One of the most critical decisions a farmer makes during a growing season is how to effectively control pests to preserve the productivity and economic value of a crop. A farmer’s reaction to seeing pests in their crops or grain is to intervene to protect his or her livelihood. The first two articles in this IPM series focused on preventative pest management [http://edn.link/prevent] strategies and observing pest populations [http://edn.link/ipm2] to determine when to intervene. This article will discuss suppressive strategies to reduce pest populations once you have decided to intervene (Figure 1). The last article in this series will address how to evaluate and assess strategies used and ways to improve your IPM plan over time.
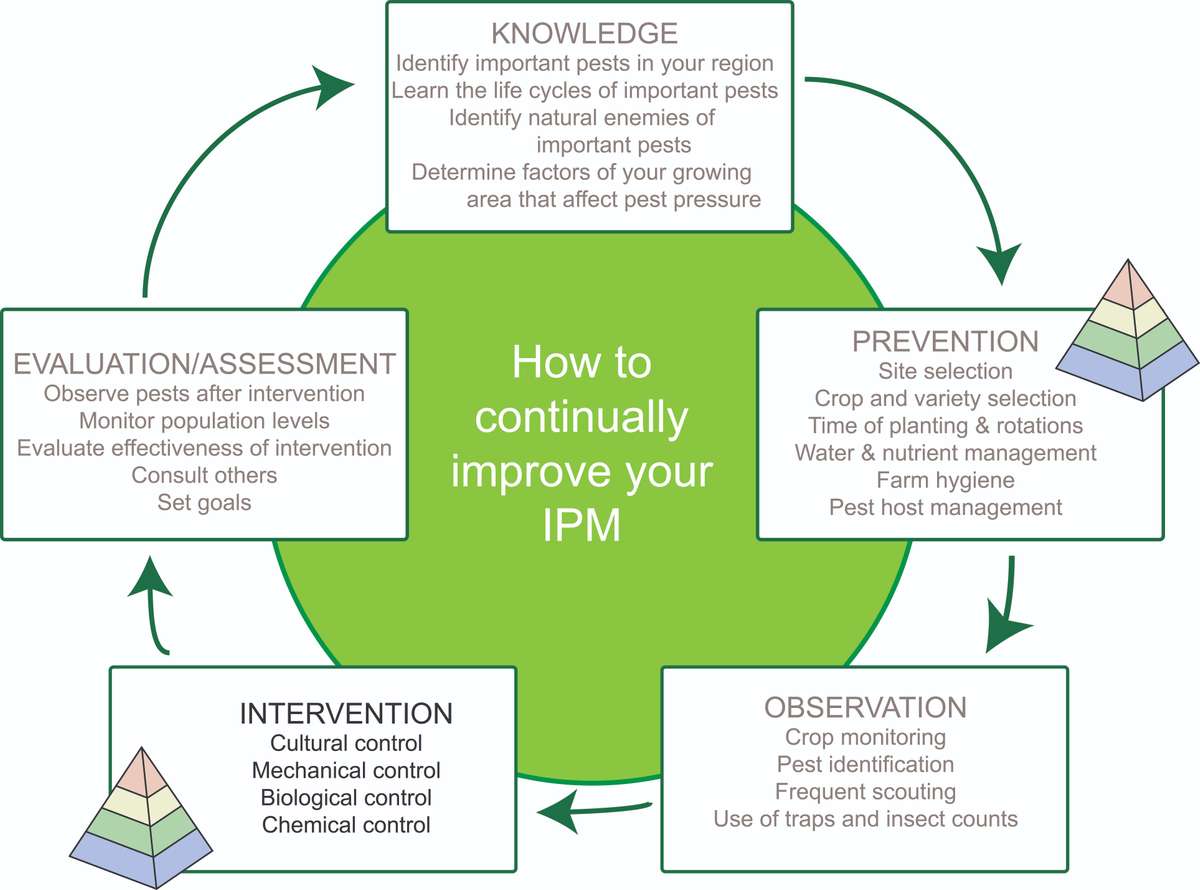
Figure 1. Stages of an example IPM cycle. Planning can start at any stage of the cycle, and the order of stages is flexible. The pyramid icon indicates strategies that prevent or suppress insect pests. Source: Adapted from farmbiosecurity, Creative Commons Attribution 3.0 license
Potential Constraints
Many regionally-practiced intervention strategies for controlling pests exist, but information is limited on which practices are effective against specific pests and under what conditions. Tesfaye and Gautam (2003) reviewed 26 traditional pest management practices used in India and Ethiopia, many of which need validation or revisiting. There is a great need for additional evaluation of locally-practiced pest control methods and the adaptation of effective pest control methods to regional contexts.
Network member Jason Weigner shared:
Anyone working with local farmers should spend time asking about local pest management techniques. Odds are you'll find things that don't really work, but you may also find some real gems. I have been researching and experimenting with ways to control leafcutters for years. Unfortunately, many things I've come up with are expensive, time consuming, or involve chemicals. Then one day I was walking with a Bolivian guy I work with looking at trees that had been defoliated by leaf cutter ants yet again and he casually said "We need to get some cotton.” The moment he told me about wrapping the trunks with cotton it made total sense. They get tangled in the cotton fibers, so they go hunting elsewhere. Such a simple and inexpensive solution found locally.
Perceptions of different control methods vary from one region to the next. Where synthetic insecticides are available and accepted, farmers are not always informed about the proper mixing and handling of pesticides, disposal of pesticide containers, and use of personal protection equipment (PPE). Moreover, pesticide handling and regulation efforts are not always consistent or enforced.
Constraints such as these will shape how farmers intervene to control pests. The remainder of this article will discuss intervention options that may help control pest populations.
Intervention Options
Always prioritize pest control options that fit the local context and utilize locally-available resources. If an intervention requires resourcing from outside the community, you should first consider other alternatives. Try to establish systems that will ensure long-term access and availability of these resources. You may need to:
- support local entrepreneurship,
- create a plan for area-wide management of the pest so that the burden falls on the whole community instead of individual farmers, or
- set up systems for governmental or organizational support.
Additionally, evaluate whether a practice will be culturally appropriate. Seek opinions and feelings of those in the community who might be affected by the pest management plan.

Figure 2. Control method categories (right) and example strategies (left) for suppressive pest control. Source: Stacy Swartz
When selecting pest control options, utilize interventions that are effective, have the least impact on the surrounding community and environment, and are complementary when integrated. When information for a specific species or crop is not available, you may need to trial potential strategies in a small area to determine which one is most effective. At times, normal seasonal changes are sufficient to keep diseases or pests in check, with no intervention other than adjusting the timing of planting. For example, diseases that flourish under humid conditions may all but disappear during the dry season. This article overviews suppressive options for situations that require intervention in response to observed pests or diseases. Pest control options are discussed under the categories mentioned in figure 2.
Cultural options

Figure 3. Potted tomato plant with minimal leaves remaining, especially at the base. Source: Stacy Swartz
Cultural controls modify the environment around the plants to make it less favorable for pests. Pests in your main crop(s) may also be harbored/hosted by other species of plants growing in or near your field or garden. Removing or pruning these alternative host plants can help minimize the pest population. If using biological control as well, preserve--as much as possible--trees, shrubs, or weeds near your crop that natural predators use for refuge, food, and nesting. You can also use cultural practices on your main crop (Table 1). Tomato plants, for example, can be managed to minimize bacterial and fungal diseases. Humidity in the leaf canopy is highest around leaves closest to the ground, which is why these water-transmitted diseases typically infect the lower leaves first and then spread upwards to the rest of the canopy. When you remove lower tomato leaves, you reduce humidity near the soil surface by increasing airflow (Figure 3). Doing so early in the season reduces early-season development of diseases.
| Strategy | Description | Example |
|---|---|---|
| Remove diseased parts/individuals | With sterile tools1, prune plant parts (leaves) or whole plants that are heavily diseased or infested. | For Cucurbita (e.g., pumpkins) with excessive downy mildew damage, you can remove leaves that are heavily infested and feed them to animals. Do not mulch with them as the pathogen may still spread. |
| Remove alternative host plants | With sterile tools1, remove alternative host plants that are heavily diseased, infested, or that host vectors. | If you are growing citrus, remove curry leaf tree (Murraya koenigii) or jasmine orange (Murraya paniculata), which are hosts of Asian citrus psyllids that spread citrus greening disease (Gast et al., 2018). |
| Use of efficient irrigation systems | To reduce water-transmitted diseases, irrigate according to plant needs and direct water to the base of plant stems. | Switch from overhead irrigation (which gets leaves wet) to drip irrigation or hand watering at the base of plants. Support plants with stakes or trellising to minimize contact of plant leaves with moisture on the soil surface. |
| 1Tools can be sterilized with isopropyl alcohol, vinegar, bleach, or high heat to kill pathogens that may be on the tool from previous use. | ||
Mechanical/physical options

Figure 4. Kaolin clay sprayed on a potted tomato plant. Source: Stacy Swartz

Figure 5. A bucket of soapy water with leaffooted bugs. Source: Annie D.
Mechanical interventions for pest control are generally categorized as passive or active. Passive options include films, dusts, oils, soaps, and traps. Films such as kaolin clay (Figure 4) can deter insects from landing on plants and/or deter feeding behaviors, but such films need to be reapplied as the plant generates new growth. Dusts such as diatomaceous earth can be placed around the base of plants to keep crawling pests from accessing the plant. Dusts can also be placed on the leaves as a feeding deterrent. Oils and soaps that kill pests are physical controls because their effect is short-term, and they act physically on the pest by smothering them or breaking down sensitive exterior tissues. Oils and soaps must contact pests and are most effective against soft-bellied insects such as aphids, mealybugs, whiteflies, spider mites, and scales. Repeated application is often required to control a population because oils and soaps are most effective at controlling young individuals. Traps are mainly used to monitor pests but can have applications for reducing pest populations (Table 2). Control of large or dense populations is difficult with traps.
Active mechanical control options include hand-picking and removing pests, vacuums or blowers (pneumatic), and hot water immersion. Picking pests off your plants by hand works well for small areas. Jason Weigner shared that:
This is a great job to get local kids to help with. I was asked in an Ayore village to help with blister beetles that were eating all their hot peppers. There were few enough bushes that we were able to get them under control quickly by turning it into a game with the kids. We made beetle grabbers out of coke bottles with soapy water in the bottom and it was a race to see who could catch the most!
You can pull or squash caterpillars by hand. Beetles, stink bugs, and other larger insects can be put into a bucket of soapy water. The soap breaks the water tension, causing insects to drown in the bucket (Figure 5). Low-tech vacuums suck pests off plants into holding containers that are later dumped into soapy water; this method is mostly used to collect specimens for pest identification. Hot water immersion effectively kills immature tephritid fruit fly (Diptera: Tephritidae) inside mango fruit (Vincent et al., 2002).
| Stragety | Description | Example |
|---|---|---|
| Picking (by hand) | Pick insects by hand and put them into a container of soapy water or shake them from the plant directly into the container. | Stink bugs (Pentatomidae family) damage tomatoes, rice grain, and much more. Handpick adult stink bugs in the morning, while they are sluggish. This practice can help decrease populations if implemented early (soon after stink bugs are detected). |
| Cultivation | Weeds are removed by scratching the soil surface with a tool. The tool either cuts the shoots from the roots of the weeds or completely buries the weeds. | A stirrup or scuffle hoe is used to cut weeds off just below or at the soil surface. |
| Traps | Traps are often used for monitoring but can also be used to control pest populations in smaller areas by removing specific life stages of the pest or reducing overall numbers. | Sticky traps in nursery or greenhouse settings can help control whitefly populations if installed early when the population is small. These are only effective against adult whitefly. |
| Other mechanical modifications | Altering the environment around plants or seeds can kill certain life stages of pests or whole pest populations. | Consider: Solar heating for cowpea weevil (Callosobruchus maculatus; ECHO Staff, 1992). |
There are also mechanical/physical techniques for managing pests and diseases in stored seed or grain. Polishing grains to remove the outer seed coat with mechanical mills helps remove pests prior to storage. Once in storage, keeping grain in sealed containers excludes pests and humidity; dry conditions prevent the growth of mold. You can also lower or replace oxygen to physically modify the environment in sealed storage containers, killing storage pests or minimizing the damage they cause. Techniques such as vacuum and replacement of oxygen with carbon dioxide are reviewed by Motis (2020) in EDN 146 [http://edn.link/lowoxygen].
Biological options “add to the answer”
Biological control reduces pests through the management of other living organisms. Biological approaches to pest control are ecologically sound, environmentally safe, and self-perpetuating. Most natural predators are species-specific and, therefore, not a threat to non-target species such as pollinator species [http://edn.link/6ryxxy]. Lastly, biological control brings stability to an agroecosystem over time; this happens as pest populations are reduced, fluctuations in pest populations are moderated, and fewer interventions are needed. Biological control alone is not likely to solve a pest problem. Rather, it supplements other control strategies, supporting long-term effectiveness of an IPM plan.
Natural predators are likely already present and active in your region. You can add a biological component to your IPM strategy by simply enabling these predators to thrive. For ideas on how to attract and sustain natural predators in your garden, read the habitat management [http://edn.link/i#habitat] section of the pest prevention article.
You can also re-introduce natural predators that are native to an area but have left the region. This process, called augmentation, is easiest to implement if supporting habitats already exist that can sustain natural predators year-round; without such habitats, the farmer must purchase and release native predators each season. The introduction of non-native predators to control local pests is called classical biological control and is too expensive and risky for most smallholder contexts. Check with local extension services to see what programs exist and what predators are available for distribution and release. Typically, government agencies, educational institutions, or other organizations are responsible for native or exotic predator research and rearing (Table 3).
| Strategy | Description | Example |
|---|---|---|
| Natural predators | A farmer keeps a few host plants for pests, plants that feed adult natural predators, and/or plants that house natural predators to encourage local populations. | Plant a few sunflowers in the off-season, which stink bugs will move to from your crop, encouraging soldier bugs (and other natural predators) to stay in the area. |
| Augmentation | An entomologist goes looking for native natural predators or parasitoids that are no longer in the region and brings them back. | Purchase or apply for parasitic wasps that have been reared by a local university, research center, or government agency. Once obtained, you can release them in your production area. An example of a gardener practicing augmentation was shared by network member Noah Elhardt. 1 |
| Classical biological control | An entomologist looks for non-native potential predators or parasitoids that, when introduced, may control the pest. Researchers rear potential insects, test their propensity to be invasive, and rule out predators that are not good candidates. | Introduction of the predatory wasp (Tamarixia radiata) to help control the Asian citrus psyllid nymphs (Michaud, 2004). This is typically too expensive an endeavor for farmers or even co-ops of farmers to conduct. |
| Microbial disruptors of midgut | Microbes that, after being consumed by a pest, produce protein toxins that create holes in the midgut of the pest. | Some Bacillus sp. are commercially sold in many places around the world and can be applied through a foliar application. Pests then consume the bacteria as they eat crop tissue. |
| Insect-killing fungi | Some fungi are entomopathogenic, meaning they parasitize and complete their life cycles on pest species. | The fungus Ophiocordyceps unilateralis completes its life cycle on only one species of formicine ant (Camponotus leonardi), making a mummy out of the ant’s exoskeleton (Shang et al., 2015). |
Beneficial organisms actively hunt and kill pests for metabolic or reproductive needs. Beneficial predators include many species of ants that consume young caterpillars (Figure 6A), aphids, and other soft-bellied insects. Prionyx wasps hunt and consume grasshoppers (Figure 6B). Beneficial parasites, known as parasitoids, deposit eggs inside a pest, eventually killing the pest (Figure 6C). The most well-known group of parasitoids are the very diverse parasitic wasps. Each species of parasitic wasp lays its eggs inside a highly specific host, which could be a soft-bellied insect such as an aphid or caterpillar or even a hard-shelled beetle. For every crop pest, there is likely at least one parasitoid targeting it. Van Lenteren et al. (2018) outline the use of many diverse species for biological control worldwide.

Figure 6. Carpenter ant (Camponotus sericeus) preying on a caterpillar (A) and predatory wasp (Prionyx sp.) preying on a grasshopper (B). Parasitized hornworm caterpillar. White, fluffy protrusions are parasitoid egg sacs (C). Source: Noah Elhardt (A & B) and Jason Weigner (C)
Biological control is a long-term investment. Parasitic wasps, for example, will not control active caterpillar populations by themselves, but they reduce numbers in future generations. That is why biological control is a vital part of IPM.
Chemical options
Chemical options, as we refer to them in this article, are those that actively work against pests through substances that are toxic to or that repel pests. Chemical control methods include both natural and synthetic pesticides 2. The active ingredient in a pesticide is the portion that is toxic or repellant. The remainder of the pesticide is inert ingredients. It is possible to select natural and/or synthetic insecticides that are “biorational” in the sense that they target specific pests, have minimal impact on the environment, and have low to no toxicity on non-target species.
Mode of action
The mode of action of a chemical intervention describes how a pesticide, whether natural or synthetic, controls the pest. Basic modes of action are summarized in Table 4.
| Mode of action | Explanation | Synthetic example(s) | Natural example(s) |
|---|---|---|---|
| Inhibitors | Inhibit pest growth, enzyme synthesis, molting, creation of chitin, or other important metabolic pathways | Organophosphates Methyl bromide Carabamates |
Decaleside (from Decalepis hamiltonii) and Rotenone (found in many plants, including Tephrosia vogelii) |
| Channel blockers | Block channels in neurological pathways or other important metabolic channels (e.g., sodium) | Indoxcarb | Tetrodotoxin (from Taricha granulosa) |
| Modulators | Keep neurological or metabolic systems open, often causing imbalances in one or more senses; a common result is disrupted feeding |
DDT Neonicotinoids Pyrethroids |
Pyrethrin Nicotine Capsaicinoids (in hot peppers) |
| Juvenile hormone mimics | Disrupt and prevent metamorphosis | Analogs of juvenile hormones | Some Echinacea spp. mimic adult hormones |
| Unknown | The mode of action for some pesticides is still unknown Some pesticides have more than one mode of action |
Azadirachtin (in neem, Azadirachta indica) |
When creating a pest management plan, select pesticides with different modes of action and time their application to vary the mode of action. Diversification of modes of action increases the likelihood of controlling whole pest populations (e.g., all life stages) and prevents pesticide resistance.3 Categories, descriptions, and examples of chemical interventions are given in table 5.
| Strategy | Description | Example |
|---|---|---|
| Natural chemicals | Chemicals extracted from natural sources. These are not always the safest and may still require PPE for application and restrictions on where and when you can use them. | Azadirachtin is extracted from neem seeds and leaves (at a lower concentration) and applied to crops to control a variety of pests, mainly piercing-sucking insects. |
| Synthetic chemicals | Manufactured chemicals. These may be more toxic or less toxic than natural chemicals. | Pyrethroid is a manufactured compound that mimics natural pyrethrins (naturally produced by chrysanthemum flowers). |
| Targeted chemicals | Chemicals specific to a species or group of insects. Such chemicals do not impact organisms outside of a narrow range of target species. | Chlorine containing carabamates such as RynaXypyr® target immature Lepidoptera, but not other insect groups. |
| Broad spectrum chemicals | Chemicals that broadly affect more than just the pest species you are trying to control. | Pyrethrins and pyrethroids kill ants, mosquitoes, moths, flies, fleas, and impact other organisms, including bees and fish. |
| Preventative application | Chemical application before a pest has been identified and sampled. Be cautious in using preventative applications as they may lead to pesticide resistance over time due to frequent exposure of the pest to the chemical. | Application of imidacloprid, a systemic pesticide (absorbed and distributed throughout plant tissue) before any pest presence is identified to ensure control of the Asian citrus psyllid, a vector for citrus greening disease (Gast et al., 2018) |
| Reactionary application | Chemical application after a pest has been identified and sampled. | Application of imidacloprid after a pest the chemical is approved for controlling (mainly piercing sucking insects) is observed. |
Safety
When using any pesticide, whether natural or synthetic, it is vital to follow correct mixing and loading practices, wear correct PPE, and practice safety measures during application. Pesticide product labels should explain the PPE required for unique settings4 that have distinct PPE recommendations. Product labels should also include any specific health risks corresponding to the product and instructions for how to decontaminate potential spills. Pesticides are sometimes repackaged and sold without labels. If a product does not have a label, try to look up the information with the product and company name, active ingredient, or commonly used name. Refrain from using a pesticide product for which you do not know the name, active ingredient, and potential health risks. Pesticide use without this information is unsafe.
Only use pesticides that are known to be effective on the target pest and apply them at a rate consistent with effective control for that pest. Application rates that vary from recommendations can cause issues with pesticide resistance, environmental contamination, or increased toxicity.
When applying pesticides, there are important factors to be considered:
- Temperature - Avoid spraying pesticides at air temperatures above 30°C, which can burn plant parts. Instead, apply in the early morning or evening when and the temperature is lower, and the sun’s rays are less direct.
- Wind - Do not apply pesticides if there are gusts or consistent wind speeds above 16 kph (10 mph). High wind speed causes your spray to drift into surrounding areas causing damage to non-target organisms, creating a safety issue, and reducing the accuracy of your application.
- Rain - Rain washes off and dilutes many pesticides. Do not apply them if it will rain soon. If it has rained shortly after an application, pay close attention to your crops to determine if reapplication may be necessary.
- Plant physiology - Younger plants are more sensitive to pesticide burns than older plants. Pesticide burns are most likely to occur when the sun’s rays are most direct, and when oils are used. If applying a pesticide with oil in it, spray early in the morning or in the evening. Flowers are more sensitive to pesticides than other plant parts, so try not to apply foliar pesticides when flowers are open.
- Proximity to water bodies - Many pesticides, whether natural or synthetic, negatively impact aquatic or semi-aquatic ecosystems. If you are close to water bodies, observe extra precautions to avoid spray drift, pesticide runoff (can occur if it rains shortly after spraying), or overapplication (can cause leaching into groundwater).
- Effect on target species - It is important to know how a pesticide impacts the target species. It may only kill or repel a specific life stage of the pest and therefore may need to be reapplied later to effectively control a population. For example, eggs are the most challenging life stage to control, and therefore you may need to reapply a pesticide once eggs of the previous generation have hatched.
- Effect on non-target species - It is essential to understand how a pesticide may affect non-target species. This consideration is important if you are hoping to create long-term health and balance in your agroecosystem. If there are harmful effects on pollinator species, biological control agents, or other organisms that are desirable in your agroecosystem, reconsider your use of the product; explore other alternatives or apply to plants before they flower (to avoid harming pollinators).
- Timing and frequency of application- Many natural pesticides are less effective than synthetic pesticides. Therefore, some natural pesticides need to be applied when pest populations are low and be applied frequently to ensure the pest population stays within acceptable levels.
If you apply a pesticide that may harm people and animals, communicate with local community members to ensure that everyone knows when and how long they need to stay out of the area. Commercial pesticides have published restricted entry intervals (REIs) that indicate when it is safe to re-enter the sprayed area.
Integration of interventions
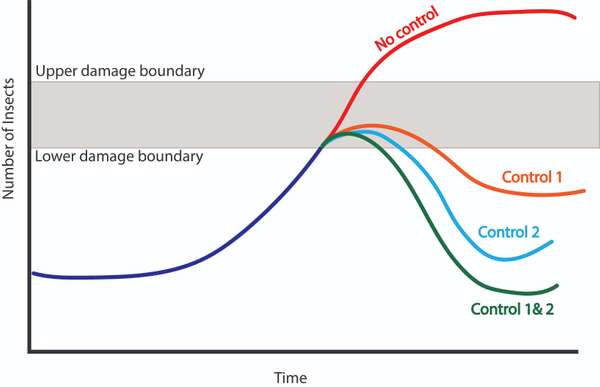
Figure 7. This diagram shows how different types of control strategies could impact a pest population. Control 1 doesn't reduce the population as much as control 2, but last a bit longer in its effectiveness. Combining both controls may have an even greater effect in reducing pest population. Source: Stacy Swartz
Cultural, mechanical, biological, and chemical intervention options should be leveraged when they are most appropriate for your context, crop needs, and level of damage. Select practices from each category that fit your needs, resources, and comfort. It is unlikely that any one practice can effectively control pests while maintaining long-term sustainability. Therefore, it is best to integrate your pest management approach with a variety of strategies that trap, repel, and reduce pests in unique ways (Figure 7). Damage boundaries also vary based on the control measures available. Jason Weigner shared a caution about suppressing pests to levels that will not sustain natural predator species:
[Reducing] the pest population too low could lead to the predator dying off or moving on [to another area], which then leads to worse pest spikes in the future.
An example of an IPM approach for dealing with sugarcane white grub in Bali, is described in Asia Note 42 [http://edn.link/qt6hxz]. Start by considering practices at the base of the pyramid (Figure 2) and work your way up if possible. If there is too much damage already done, this may not be possible, and you may have to start with stronger suppressive steps. Next season, try to invest time earlier in the season to implement cultural, mechanical, or biological options.
Conclusion
IPM is an approach to managing pests that combines different strategies, each one functioning in a unique way. Continuously improving your pest management plan requires dedication to learning about pests, observing them, and evaluating the effectiveness of your prevention and intervention actions to limit or control pest populations. In the last article, we will overview how to evaluate intervention strategies, assessing their effectiveness, and then adjust future pest management plans.
Further Reading
To learn more about natural pest control options, explore oisat.org [http://edn.link/rxj2dy], where you can navigate resources by pest, crop, or control method.
The USDA has a section on “Problem Prevention and Holistic Pest Management” specific to tropical nurseries starting on page 273 of their Tropical Nursery Manual: A Guide to Starting and Operating a Nursery for Native and Traditional Plants [http://edn.link/2mtf7j].
Rapisarda and Cocuzza’s Integrated Pest Management in Tropical Regions [http://edn.link/7x933r] book published by the Centre for Agriculture and Bioscience International is a valuable resource. The book goes deep into practices, integration of control options, and specific constraints in tropical conditions, including the unique impacts of climate change on pest management.
References
ECHO Staff 1992. Short term heating kills cowpea weevils. ECHO Development Notes no. 37.
Gast, T. and T. Watkins, summarized by Stacy Reader 2018. Yellow shoot, green fruit: citrus greening disease. ECHO Development Notes no. 138.
Michaud, J.P. 2004. "Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida." Biological Control 29: 260-269. doi:10.1016/s1049-9644(03)00161-0.
Motis, T. 2020. Low oxygen methods for insect control in seeds. ECHO Development Notes no. 146.
Shang, Y., P. Feng, and C. Wang. 2015. Fungi that infects insects: altering host behavior and beyond. PLoS Pathog. 11(8).
Tesfaye, A., and R.D. Gautam. 2003. Traditional pest management practices and lesser exploited natural products in Ethiopia and India: Appraisal and revalidation. Indian Journal of Traditional Knowledge 2(2):189-201.
Van Leneren, J.C., K. Bolckmans, J. Köhl, W.J. Ravensberg, and A. Urbaneja. 2018. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63:39-59.
Vincent, C., G. Hallman, B. Panneton, and F. Fleurat-Lessard. 2002. Management of agricultural insects with physical control methods. Annu. Rev. Entomol 45:261-281.