Preliminary findings of ECHO research
Preliminary research indicates that biochar impregnated with either MgCl2 or CaCl2 can perform well, maintaining low humidity in simulated seed storage conditions. Results suggest that these desiccants may also be useful in seed drying. Biochar successfully reduced the problem of liquefaction, commonly experienced with these desiccant salts. MgCl2 and CaCl2 are widely available through retail outlets and might be available in some low-resource areas at a reasonable cost.
Introduction
A low-cost, readily available desiccant could be very useful in the drying and storage of both seeds and food. Unfortunately, effective desiccants on the market today are relatively expensive and difficult to obtain in many low-resource areas.
An ideal desiccant should be:
- Low cost.
- Readily available, or able to be made locally from readily available materials using a simple process.
- Easily regenerated with little loss of effectiveness.
- Non-toxic and easy to handle.

Figure 8. Raw salts (foreground) and salt-impregnated biochars. MgCl2 is on the left, CaCl2 on the right. Each pile is approximately 6cm wide. Source: Tom Bierma
Some salts have desiccant properties5 and have been studied for their commercial potential. MgCl2 and CaCl2 are low cost and sold throughout the world. Non-desiccant uses range from de-icing and dust control to food and cosmetic additives. Unfortunately, both salts tend to liquefy as they adsorb water vapor, in a process called deliquescence, making them difficult to handle. As a result, their use as a desiccant has largely been limited to a few specialty applications such as research labs and museum displays.
To overcome this limitation, some researchers have applied these salts to highly porous materials, creating substances known as salt-impregnated materials, or SIMs (Figure 8). The large surface area of SIMs allows salts to adsorb water vapor, and possibly even deliquesce, while retaining a solid form, making them much easier to handle. One of the most promising SIM substrates is vermiculite. Although vermiculite is much less expensive than other SIM substrates, its use is likely too expensive for most small-scale farmers.
We explored the use of biochar as a substitute for vermiculite in making SIMs. Biochar can be made locally from almost any waste biomass and, when pyrolyzed at higher temperatures, is highly porous.
Methods
We used biochar made from bamboo pyrolyzed at 600°C in a retort kiln. Prior testing showed this biochar was not a good desiccant, achieving a moisture content of around 10% when held in a high-humidity environment for a week. Both MgCl2 and CaCl2 were obtained from local retail outlets: MgCl2 is marketed as a de-icer and CaCl2 as a food additive. Biochar was soaked in a solution of either MgCl2 or CaCl2 for 24 hours and stirred occasionally. Solids were then removed with a spoon and dried in the sun for 6 hours. This was followed by drying in a 120°C oven to a constant weight.
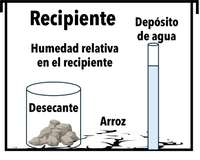
Figura 9. Recipiente sellado para la prueba del “tazón de arroz”. Fuente: Tom Bierma
The materials were tested in a simulated seed storage environment, which we refer to as the “rice bowl” test (Figure 9). Uncooked retail (from the grocery store) white rice was used as a stand-in for dried seed and placed in the bottom of a Tupperware-like container. The desiccant being tested was placed in a cup, and a narrow (~1cm diameter) water reservoir served as a source of water vapor. The relative humidity inside the container (RHc) was measured with two small hygrometers (not shown). Changes in weight were measured weekly for the desiccant, rice, and water.
In addition to the two salt-impregnated biochars, MgCl2 and CaCl2, were tested as raw salts. Two control conditions were also tested: one with no desiccant and one using common table salt (NaCl) as the desiccant. All tested conditions are summarized in table 2.
| Abbreviation | Description |
|---|---|
| No Des | CONTROL - No desiccant |
| NaCl | CONTROL - Table salt, little desiccant ability |
| MgCl2raw | Raw MgCl2 salt- no biochar |
| MgCl2BC | Biochar impregnated with MgCl2 solution |
| CaCl2raw | Raw CaCl2 salt- no biochar |
| CaCl2BC | Biochar impregnated with CaCl2 solution |
Results
Figure 10 shows that desiccants containing MgCl2 and CaCl2 maintained significantly lower relative humidity in the container (RHc) than both controls. Furthermore, CaCl2 desiccant treatments maintained a lower RHc than MgCl2 desiccant treatments. CaCl2 performed somewhat better without biochar (raw form) than with biochar. Both MgCl² treatments performed about the same. (The authors note that RHc is temperature sensitive and that testing was performed in a non-temperature-controlled space subject to wide temperature fluctuations.)
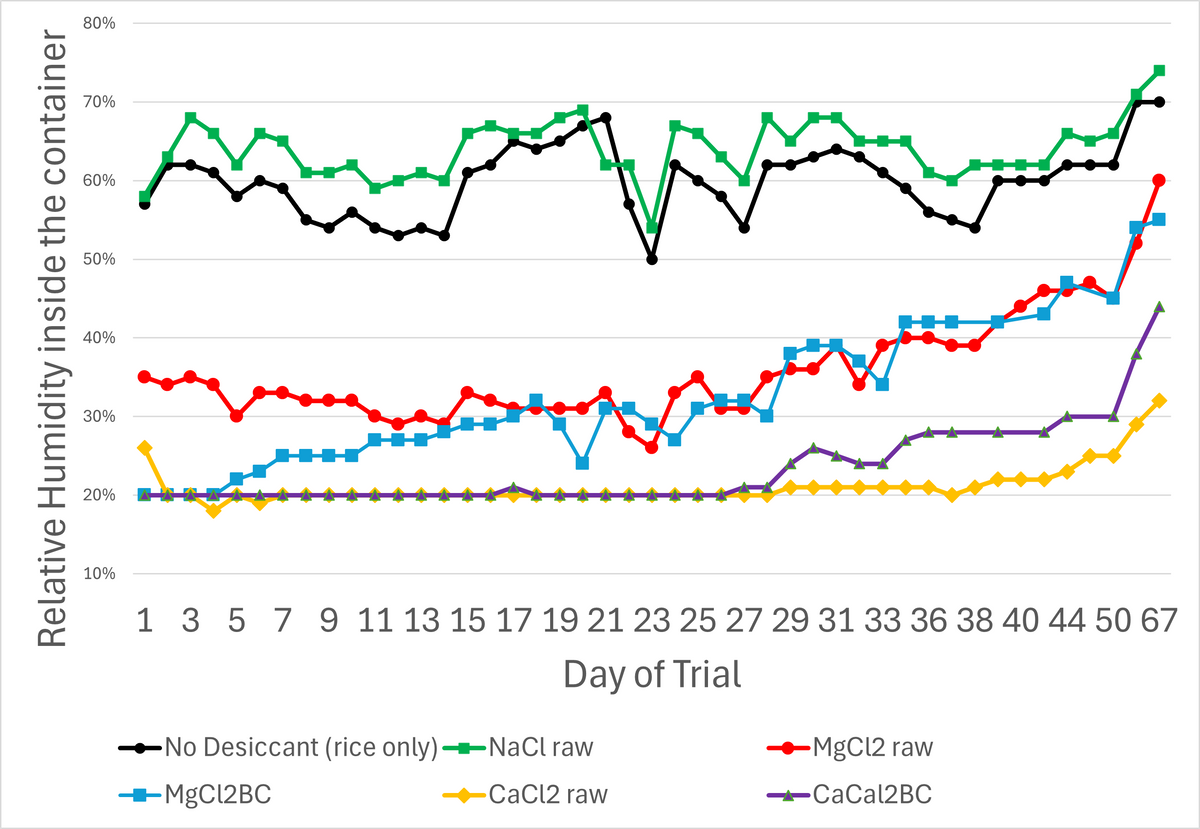
Figure 10. Relative Humidity (%) of each treatment over the duration of the trial.
Figure 11 displays the adsorptive capacity (AC) of each desiccant over the period of testing. AC is shown as the amount of water vapor adsorbed per gram of desiccant (with desiccant on a dry weight basis). NaCl demonstrated essentially no adsorptive capacity. The other desiccant treatments showed increasing adsorptive capacity throughout the tests, indicating they would continue to adsorb more water vapor even after 67 days. Again, raw CaCl2 outperformed its biochar form. MgCl2-impregnated biochar seems to have performed slightly better than the raw form.
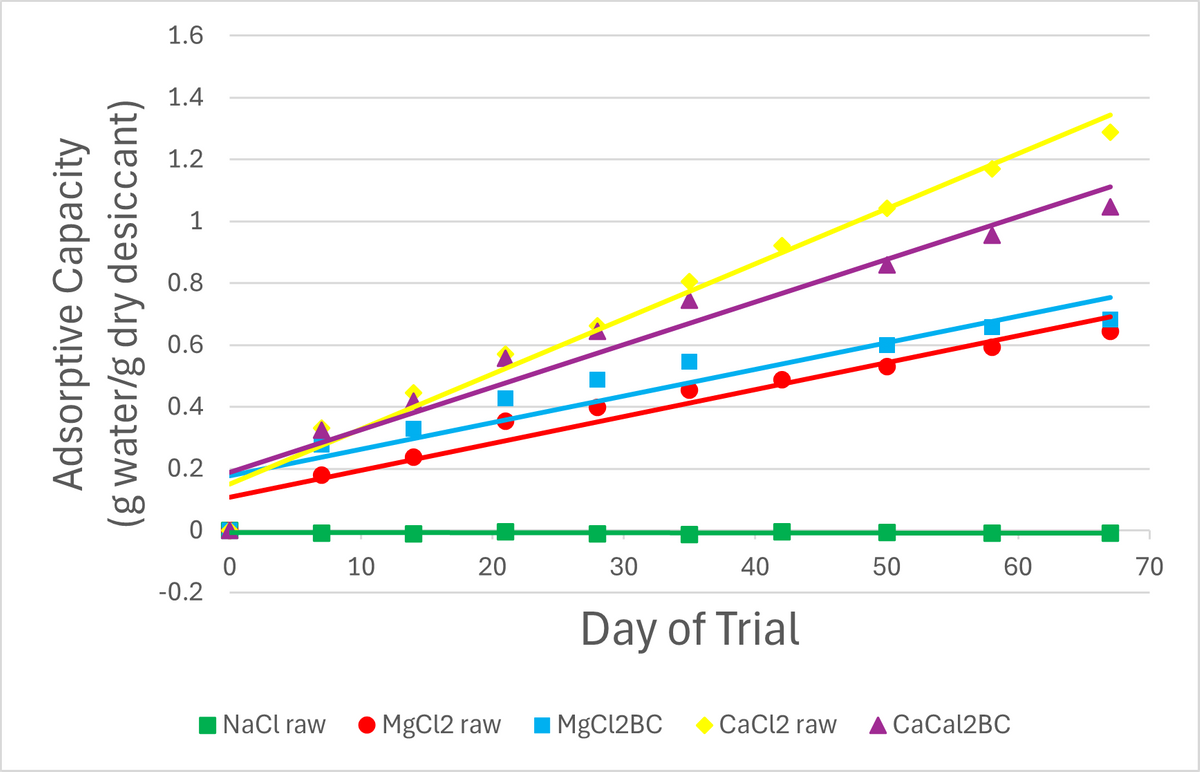
Figure 11. Adsorptive Capacity of each treatment with a plotted line of best fit (linear).
It was noted during testing that raw MgCl2 liquefied by the end of the second week (AC~0.3g/g), while raw CaCl2 remained solid for 3.5 weeks (AC~0.5g/g). CaCl2BC liquefied at 7 weeks (AC~0.75g/g), while MgCl2BC remained solid (though soft) over the entire test period (AC~0.7).
Rice weight remained essentially unchanged in the two control conditions. But rice lost 3 to 6% of its weight in the desiccant conditions during the first week of testing, even though the retail rice had already been dried to a shelf-stable moisture content. This suggests that these desiccants might be successful in seed-drying applications.
Application
Results indicate that biochar was successful in keeping MgCl2 and CaCl2 in solid form to a much higher level (AC at least 0.7g/g) compared to the raw salts (AC~0.3 to 0.5g/g). An AC of 0.7g/g is comparable to commercial desiccants such as silica gel and zeolite (AC~0.2 to 1g/g).
Both salts were successful in maintaining low relative humidities in the test containers, particularly up to an AC of about 0.5g/g for MgCl2 and 0.8g/g for CaCl2. Both salts also showed promise for seed drying applications, desiccating dry rice by 3 to 6% in the first week of testing.
Questions remaining
While these initial results look promising, additional research is needed before application in the field. Of particular importance is:
- Regeneration – How easily can a biochar SIM be regenerated, and what is the loss of efficacy with each regeneration cycle? Some preliminary tests suggest that solar drying might be sufficient for regeneration under the right conditions, but more research is needed.
- SIM production – What production method would be simple and efficient while maximizing effectiveness of the product? The current method of applying salt is somewhat wasteful and messy. It may also be possible to reduce deliquescence by reducing salt residues on the outer surface of biochar particles.
- Biochar characteristics – What biochar characteristics, such as biomass source and pyrolysis temperature, are the most important in determining SIM effectiveness? Is any biochar an adequate substrate?
- Availability and cost – How available are MgCl2 and CaCl2 in low-resource regions and at what cost? This will determine whether biochar SIMs are practical in a given area.
Further Reading
ECHO Staff. 2016. Seed storage in the tropics. ECHO Best Practice Note no. 5