Insect pests affect all forms of agricultural production, from densely planted field crops to high-value nursery plants to grains in storage. A pest management plan should start with foundational knowledge about local pest species and careful planning for pest prevention. We highlighted pest prevention strategies in the first article of this IPM series [http://edn.link/i]. After taking precautionary measures specific to your region and implementing practices that prevent pests from entering or multiplying in your production area, you will need to keep a watchful eye on pest populations and intervene before insect pests are likely to cause too much crop damage, or when they already are causing damage. This article explains some principles and practices for conducting in-field observations (sampling) to inform pest management decisions. The next article in this series will discuss intervention options and a final article will explain evaluation and assessment of intervention efforts as well as the cycle of IPM improvement.
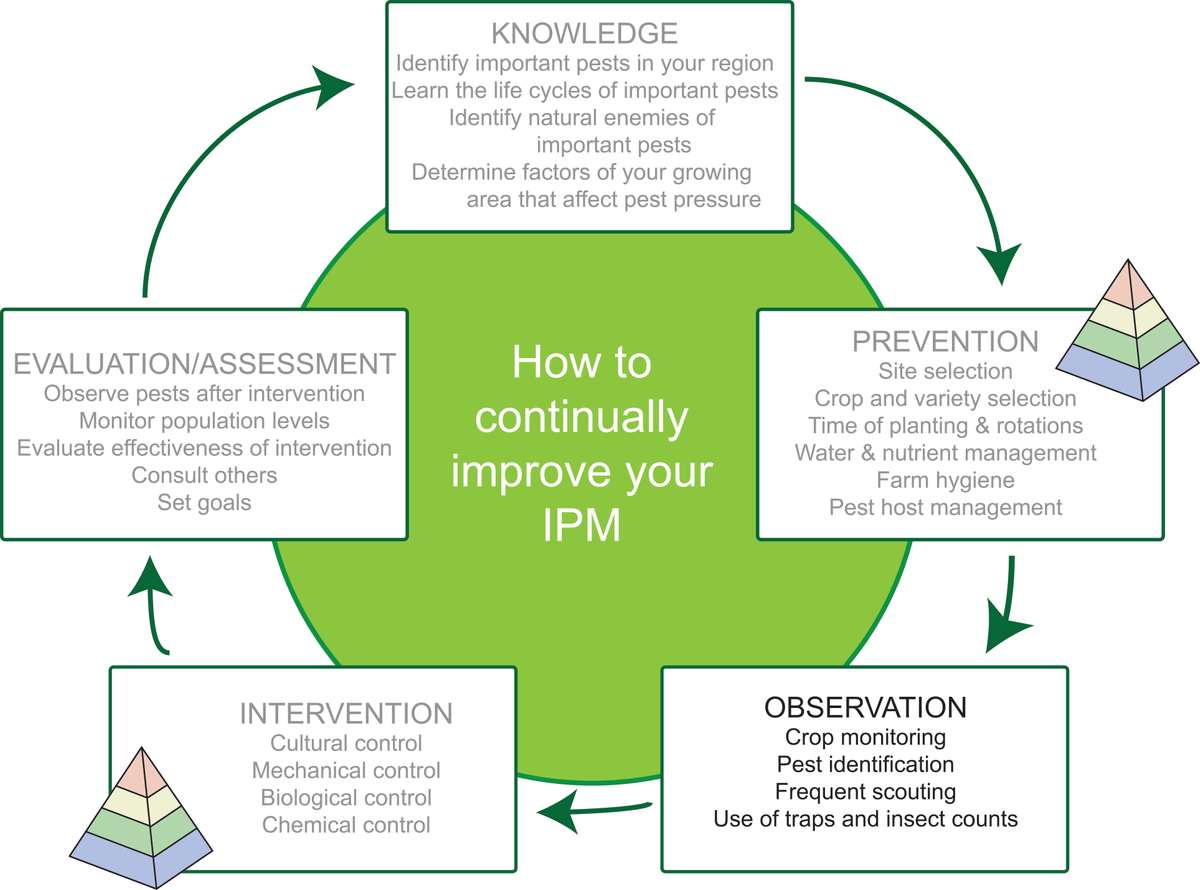
Figure 1. Stages of an example IPM cycle. Planning can start at any stage of the cycle, and the order of stages is flexible. The pyramid icon indicates strategies that prevent or suppress insect pests. Source: Adapted from farmbiosecurity, Creative Commons Attribution 3.0 license
Potential Constraints
Smallholder farmers face several hurdles that make it difficult for them to observe agricultural pests. Some farmers who have fields that are far from their home may only travel to the field for important management activities such as planting, weeding, and harvesting. This limited time in the field is sometimes insufficient to catch pest populations at levels that can be controlled in a timely and effective manner. Farmers may be unable to scout fields often enough without using valuable resources (e.g., time and travel expense).
Generally speaking, little information exists on the topic of pest management using locally available resources in tropical environments. Temperature and crop species (plant host of the pest) affect the life stage length of many pests (e.g. Nava-Camberos, et al. 2001 1). A shorter life cycle means an insect pest species can go through multiple generations and can multiply rapidly. However, little is known about how in-field tropical conditions affect pest life cycles. Also, little research has been done to better understand alternate tropical host species of many of the major agricultural pests and diseases.
When it comes to insect pest management, look for local sources of expertise. Regional agricultural universities, government agencies, or agricultural organizations may host local extension services. Trained professionals may be able to help with pest identification, early detection, and scouting practices proven to be effective in the region. Seek out these professionals’ local expertise and knowledge before taking more general approaches, such as those outlined in this article. If your area lacks such experts, you may need to depend on information or techniques that have been utilized for adjacent or similar situations.
Constraints such as the ones described in this section will shape how insect observation is practiced locally. The remainder of this article outlines general principles and practices for detecting, identifying, and monitoring pest populations, in order to inform next steps.
Observation
Globally, roughly 1 million insect species have been named. Scientists estimate that over 5.5 million more species exist but have yet to be discovered (Stork, 2018). The vast majority of insect species are beneficial or have little impact on crop production or human activity. However, a small portion (perhaps only 1% of insect species) are considered pests (Omkar, 2018). Currently, the most severe agricultural pests are non-native species that were introduced and spread through a region, for example through the import and export of agricultural products, in/on plant material, in soil, or through severe weather events. Natural predators that would otherwise keep their populations in balance may not be present outside of the insects' native areas. Sometimes, native insects that did not previously impact farmers’ crops will switch host plants to new species that farmers start to grow; this can cause the insects to be elevated to pest status. However, please understand that the majority of insects do not damage crops. In fact, many are important parts of an integrated pest management plan because they help control outbreaks of other insect species.
When insects are present in low numbers, you may find it difficult to determine whether a particular insect is a crop pest. Pests are often defined based on their feeding behavior; how quickly they reproduce; whether or not they have natural enemies present; whether they transmit diseases to humans, livestock, or plants; and/or if they contaminate the final food product. Table 1 lists mouthparts and type of metamorphosis of insect orders that include common agricultural pests. The table also gives examples of insect species from each order. The kind of mouthparts and type of metamorphosis are helpful attributes to know about, because they can indicate where an insect pest might be found, the type of damage it might cause, and the best way to treat for it.
| Table 1. Groups, mouthparts, type of metamorphosis, and specific examples of common insect pest orders. | ||||
| Order | Groups | Mouthparts | Metamorphosis | Example |
| Coleoptera | beetles weevils |
chewing | complete | Sweet potato weevil (Cylas formicarius) |
| Diptera | flies | sucking/piercing/lapping | complete | Mediterranean fruit fly (Ceratitis capitata) |
| Hemiptera | true bugs aphids planthoppers |
piercing-sucking | incomplete | Painted leafhoppers (Endria sp.) |
| Lepidoptera | butterflies moths |
chewing (immature stages) | complete | Fall armyworm (Spodoptera frugiperda) and tomato leafminer (Tuta absoluta) |
| Orthoptera | grasshoppers crickets |
chewing | incomplete | Desert locust (Schistocerca gregaria) |
| Thysanoptera | thrips | rasping-sucking | complete (modified) | African thrips (Ceratothripoides brunneus) |

Figure 2. Insect webbing is a substance insects create that sticks leaves together. This photo is of southern beet webworm on amaranth. Source: Annie D.
Metamorphosis describes the way an insect changes from an immature stage to an adult. In incomplete metamorphosis, immature insects look and act very similar to the adults. Insects that undergo complete metamorphosis go through a pupal stage where they change from one form to something completely different (e.g., a caterpillar turns into a butterfly). The immature stages of these insects typically look nothing like the adults, are often found in different locations, and can be much harder to identify. For example, many immature beetles live underground or tunnel into roots, but the adults live on the aboveground portion of the plant. Often an insect is only a pest at one stage of complete metamorphosis; many moths and butterflies do not feed as adults and can even be beneficial pollinators, but the caterpillars (immature/larval stage) can completely defoliate a crop. There will be exceptions to these categories, but understanding the basic ways insects feed and develop is critical for successful pest management.Insects’ feeding behaviors depend on their mouthparts. Insects with chewing mouthparts remove pieces of leaf or fruit tissue, leaving holes in the plant. Because these insects typically ingest plant tissue, insecticides applied to the surface of the plant may be effective. By contrast, insects with sucking mouthparts do not feed on the outside of the plant, but rather consume sap from within the plant, killing plant cells. In this situation, plant damage can include leaf distortion, yellow stippling, or shriveled grains. Since insects with sucking mouthparts only feed on internal plant juices, insecticides applied to the surface of the plant will not be effective.
Insect feeding damage, webbing (Figure 2), or other insect activity can look similar to many bacterial, fungal (Figure 3), or viral diseases or to plant nutrient deficiency symptoms. Before taking action against an assumed insect pest, verify that the symptoms you observe are not caused by a bacterial, viral, or fungal infection. Insecticides are not effective against these types of infections, and improper use of insecticides wastes farmers’ valuable resources and can kill beneficial insects. For visual comparisons of common diseases and pest damage, view this resource [http://edn.link/photoi].
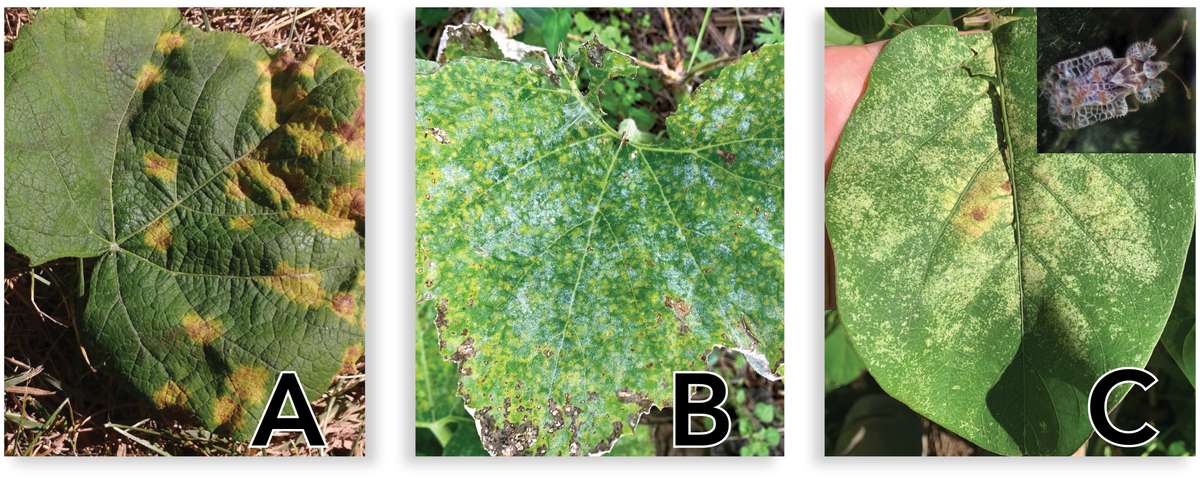
Figure 3. Leaf symptoms of fungal diseases, such as downy mildew (A) and powdery mildew (B), can look similar to damage from piercing-sucking insects. Downy mildew is restricted by leaf veins, giving it a blocky appearance with white fungal growth on the underside of the leaf. Powdery mildew has small circular discolorations across the whole leaf surface, often forming white spores on either surface of the leaf. Either of these can be confused with damage from piercing-sucking insects (C, lace bug damage on jack bean with lace bug photo inset), which cause pin-point discoloration where they have fed on the underside of the leaf. This can lead to brown necrotic spots that look similar to downy mildew. Source: ECHO Staff
Sampling
The goal of scouting is to estimate the pest population within a field or garden, rather than to count or tally the entire insect population. This sampled estimate allows you to determine whether intervening to control the pest is worth the time, money, and potential negative impacts, or whether you should wait and continue to monitor the pest population.
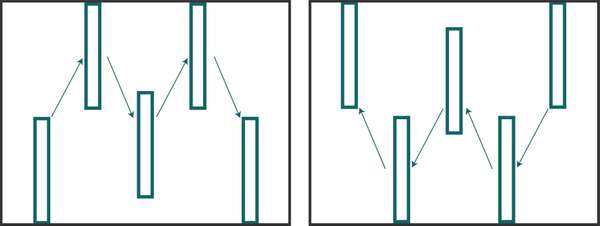
Figure 4. ‘M’ (left) and ‘W’ (right) sample pattern example. Each box would consist of several plants, each of which is inspected for insect presence or activity. Source: Stacy Swartz
You can sample for insects through active behaviors or through passive traps. For both methods, carefully consider where to sample; if you don’t, the results might be meaningless. Insect populations vary in density throughout a field, so be sure to check multiple areas in a random pattern. Many insects will congregate in one area of a field but be present in much lower numbers elsewhere. If you only sample in the heavily infested area, you might think that the pest population is much greater than it actually is. If you sample elsewhere, you might make the opposite mistake of believing that insect levels are very low, when they are not. In general, the best way to actively sample is to walk an ‘M’ or ‘W’ pattern (Figure 4) throughout the field or garden, making 5 to 10 stops to inspect plants for insect presence or activity. For example, when scouting for fall armyworm in maize, it is recommended to make 5 stops in that pattern and inspect 20 consecutive maize plants at each stop. This allows for a random sampling that represents the entire field. When scouting, also pay attention to areas of the field that might have worse damage than others (e.g. field edges often have greater amounts of damage due to points of entry from surrounding areas). In these cases, you could use intervention strategies just in those areas but not the rest of the field. For more information about how to monitor, see EDN 136 [http://edn.link/insectmonitoring] (Liptak and Motis, 2017).

Figure 5. A sweep net made from a pillowcase, thick wire, bamboo, and rubber lashing. Source: Annie D.
Active Sampling
You can accomplish active sampling in a variety of ways. Sweep netting is one way to estimate an insect population throughout an entire field. To construct a basic sweep net from local materials, take a light-colored cloth sack, add a firm wire ring around the opening (30 to 38 cm diameter), and attach it to a stick or pole (Figure 5). Sweep nets work best for low-lying crops such as rice and other small grains, or non-vining beans before they flower and fruit—plants that are tough enough to handle the damage without losing fruit or too much leaf mass. Sweep nets also work best for insects that dislodge easily from the plants.
Beating works well for plants that are too large for a sweep net. To use this technique, tap or shake individual branches over a tarp or sheet and record the number and kind of insects that fall down onto the tarp or sheet. Beating works best in cool temperatures in the early morning, when insects are more likely to fall off the branches than to fly away.
With the technique of population indexing, you indirectly measure the pest population by observing signs of insect damage. For example, you could estimate the percent leaf defoliation; amount of insect frass (feces); and/or occurrence of tents, nests, webs, emergence holes, or tunnels in fruit or stems. You could also listen for insect sounds, such as biting or chewing, to help you estimate potential insect damage.
As you scout for evidence of insect pests, be sure to gather and record data (including observations). The information will allow you to monitor changes in the insect population throughout the growing season; it can also help you know when to scout in following seasons. For an example of a scouting record sheet developed by the Canadian Foodgrains Bank, view this document [http://edn.link/faw]. Though originally used for fall armyworm, the resource can be adapted for other pests.
Some pests threaten regional food security. For these, resources may be available to monitor the spread, alert farmers, and create awareness throughout the community and region. Such resources should not replace active scouting in a particular field, but can help you know when to focus on scouting. Examples include the Fall Armyworm Monitoring and Early Warning System (FAMEWS) [http://edn.link/2gp74n] mobile app and Locust Watch [http://edn.link/6mzwhc] (for the desert locust). Both of these are available through the FAO.
Passive Sampling

Figure 6. Farmer using old yellow, plastic container and motor oil (unused) to trap pests. Source: Patrick Trail
Passive sampling typically includes the use of insect traps. Traps range from highly sophisticated and expensive pheromone traps to a container full of soapy water. When making a homemade trap, consider the color of the trap; how the insect will be trapped in or on the trap; and how often you plan to check the trap. As examples, you could use soapy water in colored pans or dishes (often yellow is the most attractive to insects; Figure 6); cards covered with a sticky material and then hung from a tree or placed on a stake in a field; or a pitfall trap made by burying a cup, so insects that walk on the ground fall inside. See this research blog post [http://edn.link/j4jfc2] for a comparison of these three types of traps deployed in a sorghum field at the ECHO Global Farm in Florida. Always add a bit of soap to traps containing water, to break the water’s surface tension. Otherwise, insects are small enough that they will stay on the surface of the water and escape. To monitor night-flying pests such as moths, you might consider a light trap. Fruit or other attractants, like meat, can make a trap more effective or can even become the trap itself. For example, in temperate regions, when monitoring for apple maggot (a pest of apples), the trap is sometimes a red plastic ball covered in sticky material and sometimes an actual apple covered in sticky material. Note that these traps are intended to help you determine the pest’s life stage, when they are active, and approximately how many are present. They are not designed to catch enough insects to provide control.
If you are growing a high value crop, you may want to purchase laboratory-prepared pheromone lures or attractants (if available) to help you monitor for specific pests.
Intervention Thresholds
One of the hardest decisions you will need to make as a farmer is to determine when a pest population is high enough that you should intervene to control the population.
The upper damage boundary (also known as the economic injury level; Figure 7) is the point at which the profit lost by the pest damage is higher than the cost it would take to intervene. (If using an insecticide, costs would include materials and the time spent mixing, loading, and spraying.) Ideally, you would never reach this point, because you would intervene earlier.
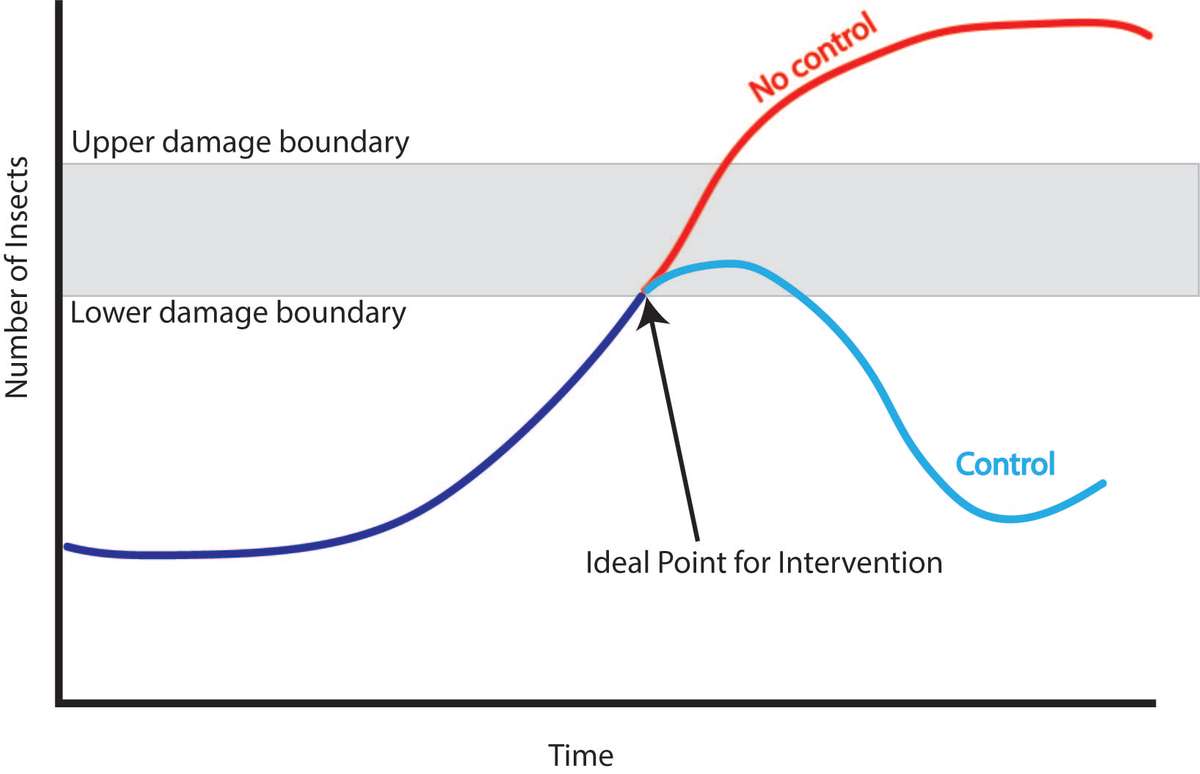
Figure 7. This diagram helps explain intervention timing. The lower damage boundary signifies when intervention should be taken to control the pest population to keep it from reaching the upper damage boundary. If no intervention is made, the pest population will likely go beyond the upper damage boundary. Source: Stacy Swartz
The lower damage boundary (economic threshold) is the action threshold. At this point, you will soon face an economic loss if you do not take action quickly. At the lower damage boundary, you need to make a decision about how to control the pest population to keep it from reaching the upper damage boundary.
The upper and lower boundary levels vary from pest to pest and crop to crop. They depend on a number of factors including crop value, location of insect damage, and crop maturity. Crop value, the most important factor, is based on financial profitability and/or the need for the crop as food for the family. Crops that are worth more have lower damage boundaries. Boundaries also vary based on what type of insect is causing the damage and where on the crop it is feeding. For example, fruit trees can generally tolerate higher numbers of insects feeding on the leaves than insects feeding directly on the fruit. Thus, the damage thresholds for fruit trees will typically be lower for insects feeding on the fruits than leaves. The age of the crop also changes the boundaries, since plants typically tolerate more damage at certain growth stages. For example, newly sprouted seeds or small transplants cannot tolerate as much damage as larger, established plants.
The presence of insects that transmit diseases (vectors) also results in lower damage boundaries. The presence of relatively few of these insects can cause significant harm, well beyond damage from feeding. An example of a vector is Bemisia tabaci, a whitefly that transmits African cassava mosaic virus.
Conclusion
Integrated pest management is an approach to managing pest control that combines many different and unique intervention strategies. To continuously improve your pest management plan, you need to keep learning about pests, observing pests, and evaluating the effectiveness of your pest control interventions. In the next article in this series, we will describe intervention options. A final article will explain how to evaluate intervention strategies, assess their effectiveness, and make adjustments to future pest management plans.
Further Reading
If you are involved with a plant nursery in the tropics, see the section “Problem Prevention and Holistic Pest Management” in the USDA’s publication Tropical Nursery Manual: A guide to Starting and Operating a Nursery for Native and Traditional Plants [http://edn.link/2mtf7j]. The section starts on page 273.
For a facilitator's guide on insect identification and monitoring, see the Canadian Foodgrains Bank's training module on Insect Identification and Monitoring [http://edn.link/9r7z6f].
References
Liptak, C. and T. Motis. 2017. Crop monitoring for early detection of insect pests. ECHO Development Notes no. 136.
Nava-Camberos, U., D.G. Riley, M.K. Harris. 2001. Temperature and host plant effects on development, survival, and fecundity of Bemisia argentifolii (Homoptera: Aleyrodidae). Environmental Entomology 30(1):55-63.
Omkar. 2018. Pests and Their Management. In: Omkar (Ed.) Pests and Their Management. Springer, Singapore.
Stork, N.E. 2018. How Many Species of Insects and Other Terrestrial Arthropods are there on Earth? Annual Review of Entomology 63:31-45.
Cite this article as:
S. Swartz and A.D. 2021. Insect Pest Management:Options for Monitoring Pest Populations. ECHO Development Notes no. 151.