This article summarizes several methods with which I have experimented to control various types of weevils in stored seed. Seed containers can range in size from small jars to barrels/drums, but must be airtight for these techniques to work. The techniques are relevant for seeds being stored for household planting or grain consumption, and can also be applied by community seed banks looking for low-cost control alternatives to chemical protectants and toxic fumigants.
Significance of insect damage in stored seeds

Figure 1. Maize seeds damaged by lesser grain borer weevils (Rhyzopertha dominica). Source: Tim Motis
Seeds are typically stored for a time before being planted or consumed. Any insects present in that seed, if not controlled, can quickly multiply and cause significant damage (Figure 1).
Many storage pests originate in the field. Seeds may appear to be free of insects at the point of harvest, but eggs and larvae from the field may still be present. Insects can also be introduced when seeds are put into previously infested seed containers or grain bins. Insect damage reduces seed germination and quality and is a major cause of post-harvest seed loss. Most insect-related postharvest seed loss is caused by various species of beetles (Order Coleoptera) and larvae of moths and butterflies (Order Lepidoptera). This article focuses on weevils, which are a specific kind of beetle.
Principles to remember
Insects die with low oxygen (O2) and high carbon dioxide (CO2)
Without enough O2, insects stop feeding and die. Insect mortality occurs at or below 5% O2 (Njorogeet al., 2019). Insects also die with high levels of CO2. According to Navarro et al. (2012), rapid mortality of a broad spectrum of insect pests occurs with 60% CO2. With elevated CO2, it is not as critical to reduce O2 all the way down to 5%.
Respiration depletes O2
As seeds and insects respire, they take in O2 and release CO2. Microbes on seed surfaces also respire; this is true, for example, of mold growth under high humidity. Respiration is an important concept behind hermetic storage, which is the practice of keeping seeds in a sealed container. With no air coming in from the outside, the O2 in a sealed bag or container declines until insect activity slows down and/or the insects die.
The rate of O2 depletion varies with seed moisture content (moist seeds respire faster than dry seeds), temperature (heat favors faster respiration), seed volume (the more seeds in a container, the less O2 there will be to start with), and presence of insects (the more there are, the faster respiration occurs). Looking at two reports, it took anywhere from <1 (Bbosaet al., 2017) to 22 (Anankware and Bonu-Ire, 2013) days for O2 in a seed-filled container to decline from 21% (the percent of O2 in the atmosphere) to 5%.
Hermetic storage can be combined with other methods
Hermetic storage is the simplest method for controlling insects in stored seed, and is appropriate for many situations. However, combining hermetic storage with other approaches can be helpful, as in the following scenarios:
- There are not enough seeds to completely fill a bag or container. As mentioned earlier, a partially-filled container has more air space—and thus more O2—than a full container.
- You have high-value seeds and want to control insects before any seed damage occurs.
- You plan to share seeds with farmers and want to take extra precautions to avoid spreading insect pests.
- You find it difficult to control insects by only excluding air. Insects’ sensitivity to low O2 can vary with life stage (Mbataet al. 2005). Eggs or pupae that might otherwise survive for a long time in a sealed container are more likely to die quickly when hermetic sealing is combined with other methods.
One approach that is related to hermetic storage is to withdraw air from a sealed container, creating a vacuum. The stronger the vacuum, the more air—and O2—will be removed.
Another approach is to displace O2 by allowing air to escape while introducing another gas into the container. In this article, we describe the use of biogas and CO2 to accomplish this; both are alternatives to chemical insecticides.
Low O2 extends the life of dry seeds
In addition to controlling insects, low O2 prolongs the life of stored seeds (Grootet al., 2015). Respiration is slowed by low O2 and low seed moisture, preserving seeds’ energy reserves. Low O2 also slows the accumulation of unstable O2-containing molecules that contribute to seed aging and deterioration (Jeevan Kumaret al., 2015). As long as they are dry, orthodox seeds will not die under low O2. (Orthodox seeds can survive drying or freezing, unlike recalcitrant seeds like avocado that will die if they dry out or freeze.)
Low O2 can be accomplished with local resources
Mason jars, jerry cans, used vegetable oil containers, and drums/barrels are all suitable containers, provided they have airtight lids or caps and are not punctured. As another option, Purdue Improved Crop Storage (PICS) bags are promoted for hermetic storage of grain; for details on PICS bags, see a publication by Uys (2017) entitled “Using airtight bags to prevent post-harvest crop loss.” PICS bags are made with high density polyethylene (HDPE), which is thicker and better than low density polyethylene bags for preventing air from diffusing in or out.
Many technologies can be used to create a vacuum, ranging from commercial vacuum sealers to low-cost devices such as brake bleeder pumps or modified bicycle pumps. ECHO TN 93 explains how to modify bicycle pumps, brake bleeder pumps, and syringes to remove at least some of the air from seed containers.
Biogas, comprised primarily of methane (CH4) and CO2, can be produced with kitchen scraps or animal manure in household-level anaerobic digesters. CO2 can be produced with materials such as organic waste, sugar, and yeast.
You can implement the methods described below with widely available materials. Use approaches that work best for your situation.
Methods trialed at ECHO
Vacuum sealing

Figure 2. Brake bleeder vacuum pump modified as explained in ECHO TN 93. Source: Tim Motis
Lawrence et al. (2017) controlled bruchids (Callosobruchus maculatus) in cowpea seeds with 600 mm Hg (mercury) of vacuum drawn with a commercial vacuum sealer. The vacuum did not adversely affect seed germination.
Building on that trial, I carried out a small experiment to find out if weevils could be controlled with a vacuum drawn by a brake bleeder pump (Figure 2). I placed maize weevils (Sitophilus zeamais) in 5 ml of maize grain into three sealed 225 ml jars under 500 mm Hg vacuum. I set up a similar treatment in another jar that was sealed but not under a vacuum. I repeated these steps with red flour beetles (Tribolium castaneum) in bird seed (mix of various grains used for this and other experiments presented in this article) and flour. Within three days, all of the maize weevils under vacuum died. Between the second and fifth week, all the red flour beetles under vacuum also died. At five weeks (present time), both types of weevils in sealed jars with no vacuum are still alive. Results suggest that, in partially-filled containers, a vacuum drawn with low-cost devices can kill insects faster than hermetic sealing alone; however, insect species vary in the time it takes them to reach 100% mortality.
How would you know if you have drawn enough vacuum to reduce O2 down to 5% or less? Percent of container volume occupied by O2 can be calculated as explained in an ECHO Research Update. With a container filled three-fourths full of maize seed, I calculated an O2 level of 3.7% with a gauge reading of 500 mm mercury.
Note that pulling air out of a partially-filled plastic bucket or similar container causes the sides to collapse inwards. That would not be an issue with “gas-assisted” hermetic sealing, where O2 is displaced by another gas. Below are some ideas for using biogas and CO2.
Biogas

Figure 3. Seed container modified with tire stems at the bottom and top of the seed container. The inner tube was connected to the bottom tire stem, which has its valve core removed to allow gas to flow into the container. The valve core was retained in the tire stem at the top of the bottle so that the valve-core pin could be pressed down (to allow air to escape while the container is being filled with biogas) and then released (to trap the biogas in the container once full). Source: Tim Motis
In a trial done in 2017, a colleague (Stacy Swartz) and I killed sawtoothed grain beetles (Oryzaephilus surinamensis) using biogas fed into an inner tube from a floating drum anaerobic digester (see ECHO TN 44) that was filled with animal manure. Squeezing the inner tube forced the gas into a 2-L plastic bottle filled with insect-infested maize seed (Figure 3). The beetles stopped moving within a few hours. Weeks later, we saw no live insects in the container; if eggs or larvae were originally present, they did not survive.
Researchers in India have already experimented with biogas for control of grain weevils (Sitophilus granarius), red flour beetles, and rice weevils (Sitophilus oryzae) that commonly infest stored rice and wheat. Based on a review of the literature and their experimental findings, Hoysallet al. (2015) drew some positive conclusions about using biogas to control weevils. First, more and more Indian farmers are using HDPE (high density polyethylene) bags/containers for seed storage, in place of traditional bamboo and adobe structures; this shift lends itself to gas-assisted hermetic storage. Second, household-level biogas systems have potential to control insects, even in partially-filled seed containers. Third, biogas does not reduce seed germination.
Some cautions are in order when working with biogas. For one thing, biogas is flammable. Be sure to monitor the tubing and connections, since gas leaks could lead to fires. For another, biogas can contain hydrogen sulfide (H2S), which is heavier than air and could conceivably accumulate in a biogas-treated seed container. At low concentrations (0.01-1.5 ppm), humans can detect the rotten egg smell of H2S (OSHA, 2020). At concentrations of 100-150 ppm, levels of H2S are high enough to cause respiratory and other problems, but H2S will be undetectable by smell--so caution is warranted at all times. H2S is dispersed by wind, so when you open a container of seeds treated with biogas, do so in a well-ventilated space with air blowing away from you. Also, consider reducing H2S by filtering your biogas through an iron oxide-containing scrubber, such as a rusty iron sponge (Vögeliet al., 2014; see pg 55).
Carbon dioxide
Below are a few simple ways to make your own CO2. If you experiment with these techniques, do so in a well-ventilated space to avoid breathing in unsafe amounts of CO2.
From fruit waste
Fruits release CO2 as they ripen and decay. With this in mind, I filled a 19-L bucket two-thirds full with star fruit (Averrhoa carambola) that had fallen to the ground underneath our trees (Figure 4). The bucket lid had a flexible rubber ring, making the bucket airtight when closed. I used a flexible plastic tube to connect the bucket of fruit to a bucket partially filled with bird seed. Within the bucket of bird seed, I placed a CO2 sensor and a cloth-covered mason jar filled with a mix of flour, bird seed, and red flour beetles. By puncturing the lid with a thumbtack, I created a small hole through which air could escape as CO2 flowed in. With CO2 being heavier than air, the air is flushed out as CO2 flows in (Saour and Yameogo, 1993). A buildup of pressure, indicated by the sides or lid of either bucket being pushed outwards, would show restricted flow.
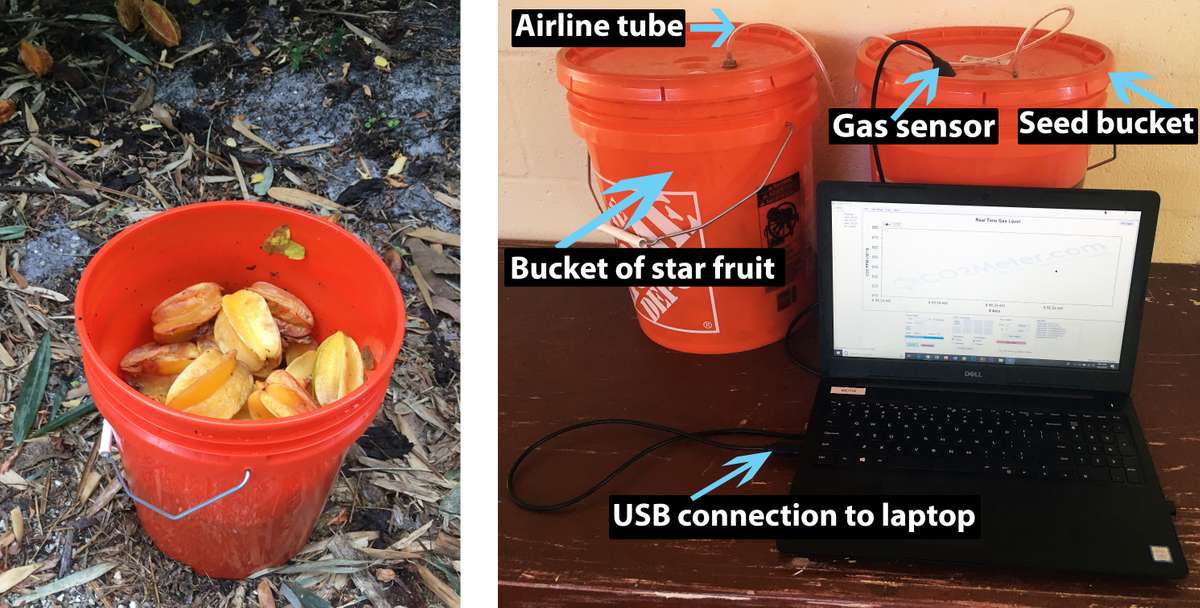
Figure 4. Bucket of star fruit (left) connected to a bucket of seeds (right), with a sensor for measuring CO2 in the seed bucket. Source: Tim Motis
The level of CO2 in the seed bucket reached 80% one week after the start of the trial. One week later, when I opened the seed bucket, the level of CO2 was still at 80% and all of the beetles were dead. This finding shows that it is possible, on a bucket scale, to kill red flour beetles within two weeks of exposure to CO2 from rotting star fruit. A potential improvement on this method would be to mash the fruit in the bucket and then add water and some commercial yeast.
From yeast fermentation

Figure 5. Barbed hose connector on bucket lid. Source: Tim Motis
Yeast added to sugar-water produces CO2. Here are some basic steps for generating CO2 this way:
- Connect tubing to the lid of an airtight container. Vinyl tubing can be pressed into a hole drilled to a diameter that is slightly smaller than the tubing. I preferred to puncture the lid of a milk jug or bucket with a nail, and then use a pliers to push a barbed hose connector (like those shown here and in Figure 5) through the nail hole and into the lid. Use putty or superglue to make a leak-free connection between the connector and the lid.
- Mix sugar with water until it completely dissolves. The amount of sugar affects the length of time over which CO2 will be generated. You need at least enough water to dissolve the sugar. I suggest filling a container anywhere from one-fourth to two-thirds of the way full. The empty space in the container minimizes liquid/water vapor from being pushed up into the tube as the yeast becomes active.

Figure 6. Baker’s yeast used to generate CO2. Source: Tim Motis
- Add some yeast. I used a dry form of baker’s yeast (Saccharomyces cerevisiae) shown in Figure 6 and commonly available in grocery stores in Florida, USA. The amount of yeast you use will influence the rate and duration of CO2 production. Experiment to optimize the amounts of sugar and yeast that will adequately sustain a desired rate of CO2 production for your needs. I suggest 1-1.5 ml of baker’s yeast for treating small jars of seed; for buckets and drums, you will likely need 15-25 ml of yeast. To prevent liquid from pushing into the tube and entering the seed storage container, place the containers so that the point at which CO2 exits the yeast-containing vessel is lower than the point at which CO2 enters the seed container (Figure 7).
- Make a small hole, the size of a pin or thumbtack, in the lid of the seed container. This will prevent pressure build-up and allow air to escape as CO2 enters.

Figure 7. Carbon dioxide from a bucket flowing into a metal drum containing maize seed. Holes for tubing and air escape were drilled into a modified lid, made with PVC plumbing parts, to avoid puncturing the drum. Source: Tim Motis
With 2.5 ml of yeast and 0.5 L of sugar, CO2 controlled cowpea weevils in a small jar (see 2017 ECHO poster). With 15 ml of yeast and 2 L of sugar, this method also controlled red flour beetles in a 114-L drum half full of maize seed (Figure 7). For the latter experiment, I placed beetles with a mix of flour and bird seed in two 50-ml plastic vials. One vial was placed at the bottom of the barrel, covered by maize grain, and the other half way up from the bottom of the drum. I opened the barrel 19 days after starting the experiment, at which time the beetles in both vials were dead. With more experimentation, the recipe could be adjusted to minimize the amount of sugar and time it takes to control weevils.
Since keeping seeds dry is important for maintaining viability in storage, minimizing the time to control insects with this method is an important goal to consider. In the above-mentioned trial with the metal drum, relative humidity in the barrel stayed near 75%. I observed no mold, a finding consistent with that of Guptaet al. (2014) who found that high CO2 levels (60%-80%) inhibited fungal growth; 80% CO2 inhibited aflatoxin (Aspergillus flavus) in their research. Even so, with CO2 generated in water or fruit waste (high in moisture), it seems best to disconnect the seed container from the CO2 source as soon as possible. Then the lid of the seed container could be briefly opened to exhaust humidity before storing (with the lid closed once again) under dry conditions (less than 65% humidity to prevent mold growth) or with a desiccant. Minimum treatment time with CO2 gas will depend on the insect species and how quickly CO2 flows into the seed container.
A simple water displacement method works well for determining the flow rate of CO2 or biogas. You will need a narrow bottle marked off in milliliters; I used a plastic graduated cylinder (Figure 8). Fill the cylinder with water and, with the palm of your hand over the top (to keep water in), invert the cylinder into a pan of water (4 or 5 cm deep). Keeping the open end of the cylinder under water, remove your hand. Water should stay in the cylinder. Keeping the cylinder upright, with the open end below the water level in the pan, place the end of the tube from the CO2 or biogas generator under the opening of the cylinder. As gas bubbles into the cylinder, the water level in the cylinder will drop. Allow a minute or two for the rate of bubbling to stabilize underwater. Then record how many milliliters of water disappear after one minute. I found that, within three hours of mixing the ingredients, 15 ml of yeast with 0.5 L of sugar dissolved in 2 L of water produced 22 ml/min of gas. Two days later, the flow rate had declined to 7 ml/min, suggesting that more sugar would have been needed to maintain the maximum flow rate over a longer period of time. Since the process produces alcohol, which becomes detrimental to yeast as it accumulates, starting with more water might help sustain a desired flow rate by diluting the alcohol.

Figure 8. Water displacement method for measuring flow rate of a gas. Source: Tim Motis
Candle burning
A burning candle consumes O2 and releases CO2 and water vapor. That made us wonder whether or not a burning candle in an enclosed, sealed space uses up enough O2 to control insects.
With a lit candle placed at the bottom of a sealed and empty 19-L plastic bucket, Stacy Swartz and I measured the resulting O2 concentration with an O2 sensor. O2 levels declined from 21% (normal for open air) when the lid was first closed to 17% when the flame went out (5 to 7 minutes depending on wick length; in a room with the lights turned off we could see when the flame went out). We repeated this three times with similar results each time. Our finding is consistent with a report by Dowell and Dowell (2017) who, likewise, found that candle burning only reduces O2 by a few percentage points.
We found that the candle produced enough heat to melt a hole in the plastic lid. That much heat would kill at least some of the insects in a container. It seems likely that the heat could also adversely affect the viability of seeds near the candle; a germination test would confirm this.
Conclusion
Our research results to date, combined with findings in scientific literature, indicate that low O2 does control seed pests and can be achieved in creative ways without expensive equipment. Hermetic sealing is already widely promoted and practiced. Benefits of vacuum sealing have also been well documented. The CO2 and biogas methods discussed in this article are not as well-tested. I was encouraged to find that red flour beetles can be controlled on a barrel scale with simple ingredients like sugar and yeast. I hope that this article will raise awareness of the benefits of low O2 for seed storage, and that the information provides insights for further innovation. Always, when trying something new, experiment to make sure it works before promoting it to farmers.
References
Anankware, J.P and M. Bonu-Ire. 2013. Seed viability and oxygen depletion rate of hermetically stored maize infested by major insect pests. Scientia Agriculturae 4:13-19.
Bbosa, D., T.J. Brum, C.J. Bern, K.A. Rosentrater, and D. Raj Raman. 2017. Evaluation of hermetic maize storage in 208 liter (55 gal) Barrels for smallholder farmers. Agricultural and Biosystems Engineering Publications 818. https://lib.dr.iastate.edu/abe_eng_pubs/818
Dowell, F.E. and C.N. Dowell. 2017. Reducing grain storage losses in developing countries. Quality Assurance and Safety of Crops & Foods 9:93-100.
Groot, S.P.C., L. de Groot, J. Kodde, and R. van Treuren. 2015. Prolonging the longevity of Ex Situ conserved seeds by storage under anoxia. Plant Genetic Resources 13:18-26.
Gupta, A., S.N. Sinha and S.S. Atwal. 2014. Modified atmosphere technology in seed health management: laboratory and field assay of carbon dioxide against storage fungi in paddy. Plant Pathology Journal 13:193-199.
Hoysall, C., P. Chandran, and H. Kumar. 2015. The efficacy of biogas to protect stored grains from insect pests. Carbon – Science and Technology 7:42-52.
Jeevan Kumar, S.P., S. Rajendra Prasad, R. Banerjee, and C. Thammineni. 2015. Seed birth to death: dual functions of reactive oxygen species in seed physiology. Annals of Botany 116:663-668.
Lawrence, B., A.J. Bicksler, and K. Duncan. 2017. Local treatments and vacuum sealing as novel control strategies for stored seed pests in the tropics. Agronomy for Sustainable Development 37:6.
Mbata, G.N., M. Johnson, T.W. Phillips, and M. Payton. 2005. Mortality of life stages of cowpea weevil (Coleoptera: Bruchidae) exposed to low pressure at different temperatures. Journal of Economic Entomology 98:1070-1075.
Navarro, S., B. Timlick, C.J. Demianyk, and N.D.G. White. 2012. Controlled or modified atmospheres. Chapter 16 in: Stored Product Protection. D.W. Hagstrum, T.W. Phillips, and G. Cuperus, Eds. Kansas State Research and Extension.
Njoroge, A.W., R.W. Mankin, B. Smith, and D. Baributsa. 2019. Effects of hypoxia on acoustic activity of two stored-produce pests, adult emergence, and grain quality. Journal of Economic Entomology 112:1989-1996.
Occupational Safety and Health Administration (OSHA). Hydrogen Sulfide. https://www.osha.gov/SLTC/hydrogensulfide/hazards.html Accessed 11 January 2020.
Saoura, S. and C.S. Yameogo. 1993. The CO2 method to control insect infestation in tree seed. Danida Forest Seed Centre. Technical note, No. 42
Uys, F. 2017. Using airtight bags to prevent post-harvest crop loss. Africanfarming.com
Vögeli Y., C.R. Lohri, A. Gallardo, S. Diener, and C. Zurbrügg. 2014. Anaerobic digestion of biowaste in developing countries: practical information and case studies. Swiss Federal Institute of Aquatic Science and Technology (Eawag), Dübendorf, Switzerland.
Cite as:
Motis, T. 2020. Low Oxygen Methods for Insect Control in Seeds . ECHO Development Notes no. 146