[Editor’s Note: This guide expands on a summary by Chalermliamthong and Trail (2021; http://edn.link/rqa3hk) of the black soldier fly larvae production system at the ECHO Asia Small Farm Resource Center in Chiang Mai, Thailand. The production system there serves as an approach for scaling up larvae production as an alternative protein source to fish and soy meals for agricultural livestock feed. The authors encourage adaptation to fit needs, resources, and constraints of your local context. The end of this article includes some considerations for household-level operations. If you have questions or experiences to share, please post on ECHO Conversations.]
Introduction and rationale

Figure 1. Close up of an adult black soldier fly (Hermetia illucens). Source: Andre Fonseca
The black soldier fly (Hermetia illucens; Figure 1), hereafter abbreviated as BSF, is native to the Americas but has spread throughout the world. Black soldier flies (BSFs) are of the class Insecta, of the order Diptera (true flies, having two wings), and the family of Stratiomyidae (soldier flies). Adult BSFs make a loud buzzing noise when flying, causing confusion with wasps (which are not true flies, having four wings instead of two and a stinger).
BSF larvae (BSFL) are grown for their protein- and fat-rich biomass that can be fed to many types of livestock. Dried BSFL contains 37% to 63% protein (with essential amino acids), 7% to 28% fat, and are a rich source of vitamins and minerals (Barragan-Fonesca et al. , 2017; Zulkifli et al. , 2022). The nutritional profile of the larvae depends on what they are fed (feedstock). Based on a review of crude protein and crude fat values by Barragan-Fonesca et al. (2017), you can obtain high values of both protein (40% or more) and fat (20% or more) with animal manure (e.g., cattle, chicken, pig), fish waste, and palm kernel meal. Larvae with close to 40% protein content are obtainable with fruit and vegetable waste, but fat values will be lower than with other types of organic waste (<10%).
With their voracious appetites, BSFL feed on decaying fruits, vegetables, animal carcasses, various types of animal manure, and more. BSFL are efficient feeders, requiring 4.5 to 10 kg of organic waste to produce 1 kg of larvae biomass (Rehman et al. , 2022). By comparison, it often takes 10 kg of feed to produce 1 kg of beef (Smil, 2002). Material that remains after harvesting larvae contains a mix of frass (larvae excrement and exoskeletons) and residue of the organic material fed to the larvae. It is often used or sold as a soil amendment.
BSFL production offers a low-cost means of converting on-farm waste into useful products. Turning waste into resources improves farmstead resilience, creates income-generating opportunities, and addresses environmental problems associated with landfills and manure accumulation. Common housefly (Musca domestica) larvae also feed on organic waste; however, they do not feed on as wide a range of materials as BSFL. Moreover, unlike houseflies, BSF adults are not a nuisance pest; they do not bite or sting and are not a bother to humans. Composting worms used for vermicompost have different uses from BSFL. For more information about vermicompost, see ECHO Technical Note 66: Vermiculture Basics & Vermicompost.
Disease risk
Decaying organic materials can carry disease-causing pathogens that can affect animal and human health. The mid-portion of the BSFL gut is known to be highly acidic (pH ≤ 3 according to Bruno et al. , 2019) and to contain substances and fungal organisms with activity against pathogens such as Staphylococcus aureus and Salmonella spp. (Gorrens et al., 2021; Zhang et al., 2022). There is evidence, however, of pathogen survival in the intestines of BSFL (Müller et al., 2019).
Much of the literature on BSF says that the adult flies do not transmit diseases since they do not bite and are said to rely on fats accumulated in the larvae stage for food. Bruno et al. (2019), however, showed that adults do in fact have the capacity to eat and digest food, raising the possibility of disease transmission.
Given that BSFs are associated with decaying organic waste, and the fact that reproductive structures of animal parasites have been found in the intestines of BSFL, disease transmission is possible (Goddard, 2003; Müller et al. , 2019). Nyangena et al. (2020) experimented with the following options for reducing or eliminating pathogens:
- Boiling larvae for 5 minutes
- Toasting over an open flame at 150°C for 5 min with regular turning of the larvae to keep the larvae from sticking together or burning
- Oven-drying larvae at 60°C until there is no more weight loss, which takes 2-3 days
- Solar-drying larvae in a box covered with clear plastic
Treatments that eliminated Salmonella in BSFL were boiling, toasting, and combinations of boiling or toasting followed by solar- or oven-drying. Drying has the added benefit of concentrating nutrients and making it possible to store the larvae. Heat treatments also reduced human pathogens in BSFL in a study by Soomro et al. (2021), with oven-drying for 22 minutes at 150°C being the most effective method.
Whether or not to do any post-harvest processing of the larvae, and which methods to adopt, depend on factors such as end use. Whereas a farmer might feed live BSFL to livestock such as poultry and fish, a much greater degree of caution is advisable if the larvae are to be used for human consumption. Many post-harvest processing approaches require an energy source, a factor that needs to be considered along with the quantity of larvae to process. See Chapter 5 of Black Soldier Fly Biowaste Processing by Dortmans et al. (2021) or ICIPE’s video on processing larvae for more information on drying methods including a small-scale heat treatment called sand roasting.
Life cycle and conditions
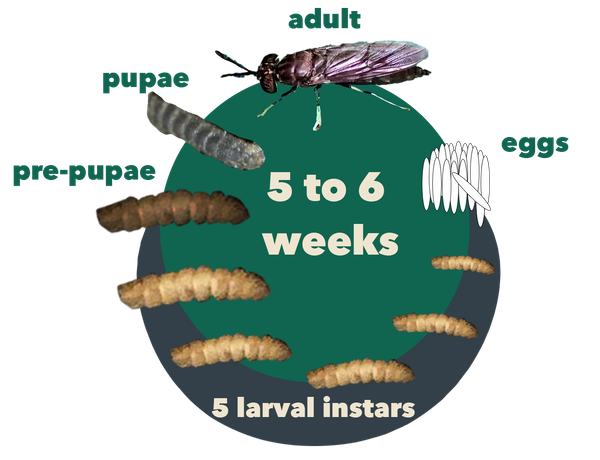
Figure 2. The life cycle of BSF, lasting about 45 days in its entirety. Source: Stacy Swartz
As shown in Figure 2, BSFs undergo five stages of development: egg, larva, prepupa, pupa, and adult (Rehman et al., 2022). Adult flies deposit eggs in dry crevices near rotting organic matter. Eggs hatch after about 4 days, producing larvae that feed on organic waste over a period of 13 to 18 days while passing through five development stages called instars. As they grow, they shed their exoskeleton between instar stages. After the fifth instar, the larvae darken as they reach the prepupal (sixth instar) stage. Prepupae stop feeding and migrate away from their food source to a dry place where they enter the pupal stage. Two weeks later, adult flies emerge from the pupae case.
BSFs prefer a warm climate. Tomberlin et al. (2009) found that BSFL survival and growth rates were highest at 27°C and that the upper range for BSF development is between 30 and 36°C. Similarly, Chia et al. (2018) found that BSF egg viability was highest (80%) at 30°C and lowest (< 11%) at 15 and 40°C. Between the minimum and maximum temperatures at which BSFL will grow, larvae develop more slowly and reach a larger size at cooler temperatures (Tomberlin et al., 2009). Conversely, they grow faster and attain a smaller size at warmer temperatures.
With respect to humidity, although BSFL have been found to tolerate a range from 30% to 90% relative humidity (Sheppard et al. , 2002), 50% to 70% is optimal (Barry, 2004). Overly moist conditions lead to reduced oxygen in the feedstock. Overly dry conditions will cause larvae mortality due to desiccation.
Reproduction in Captivity
BSFL are produced using systems that range in size from small bins, buckets, or troughs to room-size enclosures for larger-scale production. Regardless of size or scale, the basic principles and steps are similar. The system described below is that of a system used by ECHO staff in Asia that produces approximately 10 kg of larvae per week. A later section of this article will highlight concepts used in small-scale systems.
Step 1. Obtain a starter population of larvae
To start a BSFL production system, purchase larvae from a local source or hatch BSF eggs from wild flies present in your area. The latter option provides a way to start with BSFs in areas where there are no sources of larvae for purchase. By starting with eggs, if the air temperature is favorable (near 30°C), you can quickly obtain enough individuals to start a BSF colony. Tomberlin et al. (2002) observed that the number of eggs laid per female BSF ranged from 206 to 639.
Basic procedures for collecting eggs from wild BSF are as follows:
A. Prepare an attractant. Options for an attractant include rotting fruit, kitchen scraps, fermented bran (outer layer of grains removed during milling), and manure. Lamin et al. (2022) found that rotten pineapple worked better than rotten bananas or fermented bran as an attractant. They attributed the success of the rotten pineapple to its strong smell. Using rotten fruit as an attractant involves crushing it to obtain a creamy texture, adding water as needed, and placing it into a bowl or basin. If you use bran, ferment it by mixing it in a 1:1 ratio with water, letting the mixture sit for 3 days, and then combining it with mashed bananas (see YouTube video by Adi in Indonesia entitled Best Attractant: Fast and Easy Way to Get Seeds of Black Soldier Fly).
B. Place the container with the attractant in an outdoor place where BSF are likely to be.

Figure 3. A container filled with rotting fruit, with stacked wooden blocks on top, to attract adult black soldier flies for purposes of egg collection. Source: Tim Motis
C. On top of the container, provide a surface for female BSFs to lay their eggs. Surfaces with cracks and crevices work well. A good way to do this is by placing several stacks of wooden blocks over the container. Create each stack by cutting pieces of wood to a length that exceeds the diameter of the container. Stack several pieces of wood and then secure the stack with a rubber band around each end (Figure 3). As suggested by ECHO Asia staff, you can place toothpicks between the pieces of wood to create space between the layers of wood for adult female flies to lay their eggs.
D. Monitor the stacks of wood for eggs. If you don’t see eggs, it could mean that wild populations around the attractant are non-existent or low. In that case, try placing the attractant where there are likely to be more wild BSFs. Look for a place with a lot of organic waste, such as near waste dumps. If you are in a location that is not sunny and warm all year, keep in mind that BSFs may not be active during cool winter months.
E. Hatch the eggs to obtain larvae. When you are starting a BSF colony from wild BSF eggs, the mating enclosure, described in the next step, is a good place to hatch the eggs. Thereafter, you will be able to produce eggs in the mating enclosure instead of having to collect them outside from wild BSFs.
Step 2. Establish a mating enclosure

Figure 4. The screened-in BSF mating enclosure includes overhead sprinklers for moisture control. Here adult flies mate and females lay eggs. Source: ECHO Asia
Establishment of a mating enclosure is necessary for producing eggs and BSFL. Mating enclosures can range from large screened-in rooms, such as the one pictured in Figure 4 from the ECHO Asia Farm, to smaller systems using mosquito nets, or even mesh baskets. Regardless of the scale or design, the mating enclosure must maintain adequate moisture (around 70% relative humidity) and a temperature of 24 to 38°C 1 while keeping the BSF in, and pests out.
Make provisions for adult flies, including a water source and some vegetation or other surfaces on which to hide and mate. A sugar source like honey will prolong the lifespan of adults (Barragan-Fonseca et al. , 2017). The pupae will not eat any food, so you need only a small amount of food nearby to entice adult female flies to lay eggs.
Step 3. Collect the eggs

Figure 5. Inside the breeding enclosure, provide wooden blocks with small crevices for the female BSFs to lay their eggs. Blocks are placed above a food source, but not directly in contact. Adult females lay eggs in cracks between wooden blocks. Source: ECHO Asia
To collect the eggs of the adult female BSF, provide favorable material for laying eggs within the mating enclosure. The ECHO Asia Farm discovered that small blocks of wood work well and provide both an inviting egg-laying environment and easy egg collection method for the workers. Using small pieces of cardboard may be logical (Wong, 2020), but the wooden blocks are convenient for collecting eggs and result in higher quantities of eggs as experienced by ECHO Asia staff. At this stage, it is important to note that BSF do not lay their eggs directly on (or in) a food source, but nearby to one. Blocks should be near a food source, as shown in Figure 5.

Figure 6. Once females lay eggs, remove the wooden blocks and take them apart for easy access to the eggs. Toothpicks are used to separate blocks, providing small gaps for females to lay eggs. Source: ECHO Asia
To collect eggs (Figure 6) remove the blocks, separate them from each other, and carefully scrape off the eggs with a toothpick or other small, pointed item. Eggs can be of different ages if workers do not remove the blocks each day. By having eggs of different ages, the larvae will hatch and grow at different stages, requiring added sorting and separation before maturity. It is helpful to have larvae at uniform age and maturity when producing larger batches of BSFL.
Step 4. Transition from eggs to larvae

Figure 7. Place collected eggs gently on top of a food source, using screen mesh to avoid direct contact of the eggs with the moist food. Once eggs hatch, larvae will migrate to the food source. Source: ECHO Asia
Transfer the collected eggs to a proper food source (see step 5) where they will hatch and crawl to the feedstock provided. This could be in a separate area. Use a mesh screen to separate the eggs from direct contact with the food source (Figure 7). Eggs will hatch within four days of laying. At this stage, while larvae are small, you can use plastic trays to hold small quantities of feed/waste and larvae. BSFL will feed to a maximum depth of about 20 cm, so ideal bin depth is at least 20 cm but no more than 60 cm (Barrios, 2019).
Step 5. Select a proper feedstock
One advantage of BSFL is their ability to consume many types of waste: municipal wastes with high organic matter, market waste (fruit & vegetable), human and animal manures, table scraps, bone meal, and most other products (Figure 8). This article will not give a prescriptive list of feed sources but encourages the producer to find the so-called ‘waste’ resources locally available. Use low-cost, or even free, waste by-products including market waste, cafeteria food scraps, rice bran, spent grains, soy cake, etc. BSFL prefer foods high in fats, proteins, and starches. Table 1 points out factors to consider in selecting feedstock.

Figure 8. Food source options for producing BSFL include selecting waste like pineapple waste and byproducts collected from the market. Source: ECHO Asia

Figure 9. To make sure that BSFL grow strong and healthy, mix higher quality waste byproducts such as rice bran and soy meal, with lower quality waste byproducts. Source: ECHO Asia
As alluded to in Table 1, you can mix waste resources together to ensure a balanced, or ‘complete’ feed source (Figure 9). This helps ‘bulk up’ the feedstock to ensure higher yields of larvae produced. For example, you could boost the protein content of plant waste by adding chicken manure. You should not feed diseased animals or their offal, wood chips, pesticide contaminated items, or too much highly fiberous vegetable matter (Barrios, 2019). Experiment with various feedstocks to find out what works best for you.
| Organic waste type | Benefits and/or tradeoffs | Literature source |
|---|---|---|
| Fruit and vegetable waste | Less of an odor than rotting meat. May be low in protein and fat, which can delay larvae maturation time and weight | Nguyen et al. (2013) |
| Fish waste | High in fat but fish can also accumulate heavy metals toxic to BSFL | Nguyen et al. (2013) |
| Kitchen scraps | Well-balanced, containing both plant and meat waste | Nguyen et al. (2013) |
| Fibrous waste like almond hulls, rice straw | Increases oxygen for the larvae but the high carbon to nitrogen ratio reduces larvae weight; can add nitrogen-rich plant waste to lower the carbon to nitrogen ratio | Palma et al. (2019) |
| High-moisture feed | Too much moisture can lead to anaerobic conditions and reduced larvae growth; can mitigate with ventilation and incorporation of high-fiber materials | Yakti et al. (2023) |
| Livestock and poultry manure | Protein content higher with manure from omnivores (e.g., chickens, which eat plant and animal foods) than herbivores (e.g., cattle, which graze on grasses) | Xiao et al. (2020); Chen et al. (2003) |
Step 6. Scale up production

Figure 10. A scaled-up BSFL system in northern Thailand using individual bays with different waste sources to grow BSFL at various stages of their life cycle. Source: ECHO Asia
As larvae hatch and feed, they need to be ‘scaled up’ into larger containers or bays for adequate production. During this step, provide more feedstock for larvae to eat (Figure 10). How much feed to add will depend on larvae instar, temperature, feedstock, and other factors. Check larvae at least once a day to ensure there is always an adequate food supply for growing larvae. When you add new feed, make sure to mix the old feed and new feed evenly throughout the bay/container or add old feedstock and larvae on top of a bin with new feedstock. Larvae are photophobic (light fearing) and will move downward to the new feedstock.
***NOTE: It is critical to control moisture to avoid foul smells from rotting feedstock. Piles of feed or waste should never be anaerobic (lacking oxygen). Trays or bays should have a way to drain excess moisture to avoid any standing liquid. 2 Good drainage will also help to keep the feedstock from attracting pest flies such as the common house fly; combine good drainage with adequate coverage (lids or screen) to prevent house flies from laying eggs in the food source and infecting the BSF colony. The ECHO Asia Farm uses a dry material, such as rice bran or rice powder, to absorb moisture as needed.
Step 7. Harvest the larvae

Figure 11. Sorting and sizing BSFL. Source: ECHO Asia
Over a period of 13 to 18 days, larvae will feed voraciously, eating twice their own body weight each day (Shishkov et al. , 2019). There are a few different ways to harvest larvae.
Harvesting larvae before reaching maturity (shortly before the pre-pupae stage) requires sorting, sizing, or separation of larvae from their feed material. For larvae that are fed directly to livestock, removal from the feed source is unnecessary. Sorting and sizing are common for larger production systems but these activities are labor intensive. For easier separation, we recommend the following options:
- By the end of their production cycle, transfer larvae to a finer-textured feed source (e.g., bran). Uniformly small food particles will then be easy to separate from the larvae.
- Use various sizes of screens to facilitate sorting and sizing.

Figure 12. An example of harvested, sorted larvae. Source: ECHO Asia
Two approaches to sorting larvae for uniform size are wet and dry harvesting. Wet harvesting involves using water to carry larvae through larger screens and push smaller food debris through finer screens to isolate larvae for feed. Dry harvesting is accomplished by moving or shaking the mixed contents screens until larvae are isolated from large and small food particles (Figure 11). Workers can sort by hand or use mechanized shakers, like technologies used in vermiculture systems. Once larvae are harvested and sorted (Figure 12), they can be processed for later use as feed.
Step 8. Raising Pupae for Reproduction

Figure 13. A BSFL ‘self-harvesting’ bay where larvae that have reached the pupal cycle, will crawl away from their food source, and fall into lower troughs for collection.
Source: ECHO Asia
The ECHO Asia Farm’s ‘self-harvesting’ system (Figure 13) works very well for resupplying the mating enclosure. At the pre-pupal life stage, BSF migrate from their food source in search of a dark, quiet place to transform into a mature fly. If checked regularly, it can be convenient, and provide a steady supply of pupae for reproduction. Transport these pupae to the mating enclosure before flies emerge.
Smaller systems

Figure 14. Bucket system for small-scale production of BSFL. Plastic bin used to catch self-harvesting pre-pupae. Source: JC Barrios
There are many examples online of small, household-level systems for rearing black soldier flies. These make use of bins, buckets (Figure 14), and troughs (Figure 15). With all smaller systems, make sure to have a covering over the system to protect BSFL from rainfall events and predators.

Figure 15. Trough built for BSFL production inside of chicken coop. Source: JC Barrios
When using a bucket or bin, leave holes in the lid large enough to allow wild BSF females to enter the bucket and lay eggs. If you are in a rainy climate, you can attach PVC in a “T” coming out of the bin to provide a way for females to enter the system without getting the feedstock wet. Pieces of cardboard can be stacked in layers and attached to the top of troughs, buckets, or bins to provide a place for wild BSF females to lay eggs. If using bins or buckets with lids, females may lay eggs between the lid and bin edges. Scrape these eggs into the feedstock every three to four days. One advantage of using stacked buckets or bins is that you can drill holes in the inside bucket or bin and catch leachate for later use in an outer bucket or bin. Alternatively, you can place a catchment system under the drainage holes to collect leachate (Figure 16).
Many smaller BSF systems use the ‘self-harvesting’ nature of the BSFL for collection. As seen in the example (Figure 16), set-ups funnel the crawling pupae out of the food source. You can put your system inside of your livestock enclosure to allow livestock to immediately feed on the fallen pre-pupae (Figure 15) or you can place a container with untextured sides (e.g., plastic) under the edge of the ramp to catch the larvae. With this practice some escape is inevitable, but escaped individuals help support the wild population of BSF.
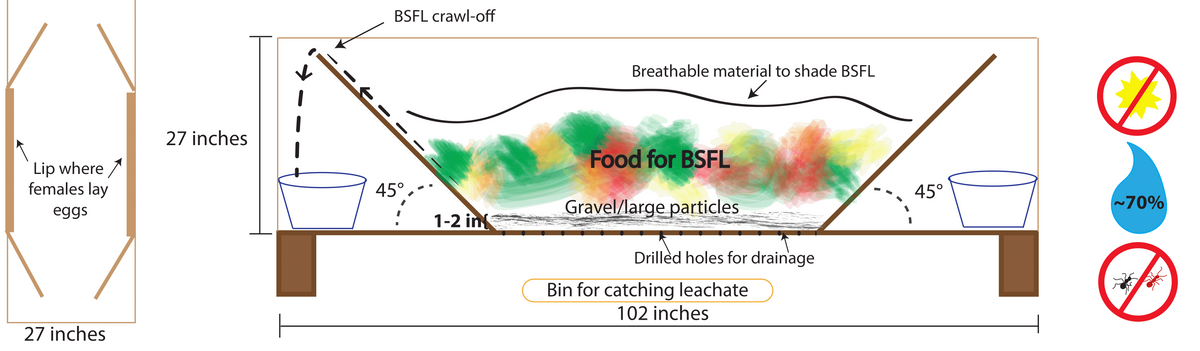
Figure 16. Diagram for trough system that includes leachate and pre-pupae catchment systems. Source: Stacy Swartz
With smaller systems, you might have to invest in additional predator defense to keep vermin and other scavengers out of the bins/troughs. ECHO Florida staff have used wooden boards weighed down with cement blocks to exclude raccoons and rats.
Production Challenges to Consider
Pests
Designers should consider pests such as birds, rats, and other possible pests before establishing a BSF system at any scale. Enclosed systems are necessary to keep BSF in and unwanted pests out. Unfortunately, the necessary process of installing screens and nets can become expensive and adds significantly to a producer’s bottom line.
Foul odors
The success of any BSF system depends on moisture control. Many food wastes, such as fruit scraps, have high moisture contents and can cause systems to become anaerobic. Preventing anerobic conditions is crucial to the overall success of the system and to the perception of neighbors and clients. Install drainage options or use brans or flours to absorb excess moisture. If you see clumps of feed, break them up to help the larvae consume the feed source.
Premature migration of larvae
If there is not enough feedstock, larvae will attempt to leave the system, crawling out of enclosures in search of food. To mitigate this migration, check on larvae frequently to ensure adequate food supply.
Using Black Soldier Fly in Livestock Feeds
Poultry
BSFL have been shown to be a nutritious supplement to animal feeds. Meal made from BSFL serves as an alternative to soybean meal and fish meal as a source of protein in poultry diets (Fanatico et al. , 2018). This has been demonstrated for chickens raised for egg (layers) and meat (broilers) production. Below are some of the reports we found in the literature for layers and broilers:
- Layers: Replacement with BSF meal of up to 100% of the fish meal used in poultry feed has been shown to increase body weight and egg production of laying hens (Attivi et al. , 2022; Zhao et al. , 2022). Studying free range hens, Ruhnke et al. (2018) found that chickens offered dried BSFL consumed approximately 15 g of BSFL per day, amounting to 16% of their diet. They suggested that, with attention to feed formulations, BSFL could be a viable long-term protein supplement, noting that drying BSFL at high temperatures (90°C) may reduce availability of some amino acids (lysine, arginine, and threonine).
- Broilers: BSFL meal has been fed to poultry with beneficial effects in various stages of broiler development (de Souza Vilela et al. , 2021). Cheng et al. (2023) found that broiler chicks put on a statistically similar amount of weight with dried BSFL as with fishmeal and soybean meal. Moula et al. (2018) showed that weekly weight gain of local poultry improved when 8% of a standard commercial feed was supplemented with whole, fresh BSFL that were thawed after having been frozen.
Incorporating BSFL into poultry feed provides chickens with a more diverse diet, which is good for the birds’ overall health. The study by Moula et al. (2018) showed that benefits of BSFL (e.g., weight gain) could be achieved with larvae reared on small systems that work well in the context of smallholder agriculture.
Aquaculture
The decline in catches of wild fish is both raising prices and stimulating aquaculture to produce more fish. Fish meal and agronomically produced vegetable proteins such as soy cannot adequately sustain increases in aquaculture. BSFL have shown promise as a viable replacement for soymeal and fish meal. Replacing 30% of grain meal (from soybean, corn, and wheat) with BSF frass increased tilapia fish weight and disease resistance in comparison to grain meal alone (Yildirim-Askoy et al. , 2020).
Zarantoniello et al. (2020) pointed out that BSFL meal is often high in saturated (unhealthy) fats and deficient in polyunsaturated (healthy) fats. To boost polyunsaturated fats of larvae fed with coffee grounds, Zarantoniello et al. (2020) added freshwater microalgae (Schizochytrium sp.) to the feedstock. They found that larvae fed with a combination of coffee grounds and microalgae increased polyunsaturated fats in BSFL and that the resulting BSFL meal could replace up to 50% of fish meal in fish feed.
BSFL can also be used in aquaponics, a form of aquaculture in which fish farming is linked with plant production. The plants in aquaponic systems utilize nutrients from fish waste. Romano et al. (2022) found that frass from BSFL, made into a dilution or tea (at a rate of 0.09 g per 40 ml, with the resulting 40 ml solution added to each 1000 L tank used in the trial), increased levels of calcium, potassium, and phosphorus in the water. They mentioned that calcium, potassium, and iron are often limiting for plant growth in aquaponic systems.
Pigs
Pigs have been found to prefer live BSFL over other foods like maize and commercial feed pellets (Ipema et al. , 2021). Pigs will also eat BSFL that have been dried and ground into powder to make BSFL meal. BSFL meal serves as a good protein source for pigs and boosts their intestinal health (Kar et al. , 2021). BSFL may replace at least a portion of other protein sources like soy and fish meal, without any adverse effects on growth or quality of pork (Hong and Kim, 2022).
The rearing of BSFL also provides farmers with a way to dispose of pig manure. Parodi et al. (2021) found that BSFL incorporated—into their bodies —12% of the carbon and 25% of the nitrogen contained in the fresh manure that they were reared on. Storing nutrients in BSFL keeps them from being lost to leaching, thereby mitigating against groundwater pollution associated with accumulation of fresh manure.
Ruminants
Insect meals may be used to alleviate shortages in fishmeal and plant-based protein supplements for ruminants (Castillo and Hernández, 2023). Jayanegara et al. (2017) showed that the high chitin content in BSFL lowers digestibility, suggesting that this be considered in developing livestock feed formulations. On the other hand, their work showed that high chitin content is linked to reduced rumen emission of methane (a greenhouse gas). BSFL can be used to supplement the diet of young livestock that are still nursing and as a source of probiotics(Astuti et al., 2022).3
Using byproducts for fertilizer
Frass or larval insect excrement is a by-product of insect rearing and can be used in field or nursery settings. BSF frass can be soaked in water to make a ‘compost tea’ that can be applied as a soil drench. Frass can also be added to potting mix or placed at the bottom of furrows before seeding crops directly into the soil. Nutrient values for BSF vary based on feedstock. Researchers Lopes et al. (2022) reviewed various studies and found that frass from BSFL fed vegetables contained N-P-K (nitrogen-phosphorus-potassium) values of 2.8-1.5-3.3, compared with larvae fed cow 1.9-1.0-0.2, poultry 2.3-1.1-1.8, and pig 2.4-2.1-1.0 manures. Like other fertilizer alternatives or soil amendments, it is a bulky practice requiring 3,571 kg of material for 100 kg of N. The incorporation of BSF frass as a soil amendment has additional benefits such as improvements in soil structure, increased soil organic matter, and water holding capabilities.
If you are facilitating drainage in your system, leachate collected can also be used as fertilizer. Collected leachate can be diluted and used as a fertilizer. Green (2013) observed improved plant growth with BSF leachate diluted in water (e.g., 1 part BSF leachate mixed with 9 parts water) and applied as a soil drench or foliar spray.
Final thoughts
BSFL production provides farmers with a way to obtain protein for animal feed, process and recycle on-farm waste, and generate by-products such as frass and leachate for soil improvement. Systems range in scale from small bins and containers to larger-scale systems with dedicated mating enclosures. Both small and larger-scale approaches can be implemented with commonly available materials.
While the steps in raising BSFL are not difficult to implement, it is important to understand factors that influence the growth and quality of BSFL. Some factors that have been identified in this document include temperature/humidity, feedstock, feedstock characteristics such as moisture content and depth, and processing options. A farmer will also need to consider economic factors such as feedstock availability, the quantity of larvae needed, feasibility of processing, and cost of materials.
Additional Resources
ECHO Asia virtual training on BSF Production
Information from the Critter Depot on:
Life stages of black soldier flies: https://www.thecritterdepot.com/blogs/news/nutritional-benefits-of-black-soldier-fly-larva-frass-critter-depot#:~:text=The%20frass%20sold%20by%20The,types%20of%20manure%20and%20compost
Nutritional composition: https://kimmyfarm.com/en/nutritional-value-of-black-soldier-fly-larvae#sun-dried-bsf-black-soldier-fly-larvae-fresh-high-quality
Tips for feeding chickens: https://www.backyardchickens.com/articles/feeding-chickens-an-introductory-guide.67139/
Insect meal as animal feed: https://www.feedipedia.org/content/insect-meals-animal-feed
International Centre of Insect Physiology and Ecology (ICIPE) Black Soldier Fly Video Series (6 episodes): https://www.youtube.com/watch?v=-8vejnFgndk
Cite as:
Chalermliamthong S., P. Trail, R. Walle, and T. Motis. 2023. Black Soldier Flly Larvae Production. ECHO Technical Note No. 99.
References
Astuti, D.A. and K.G. Wiryawan. 2022. Black soldier fly as feed ingredient for ruminants. Animal Bioscience 35(2):356-363.
Attivi, K., K.G. Mlaga, K. Agboka, K. Tona, Y.A.E. Kouame, H. Lin, and K. Tona. 2022. Effect of fish meal replacement by black Soldier Fly (Hermetia illucens) larvae meal on serum biochemical indices, thyroid hormone and zootechnical performance of laying chickens. Journal of Applied Poultry Research, Vol. 31(3) 100275.
Barragan-Fonseca, K.B., M. Dicke, and J.J.A. van Loon. 2017. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed – a review. Journal of Insects as Food and Feed. 3(2):105-120. https://avingstan.com/wordpress/wp-content/uploads/2019/08/Barragan-Fonseca-et-al-2017-Nutritional-value.pdf. Accessed 13 Jan 2023.
Barrios, J.C. 2019. Black soldier fly larvae: Biology and life cycle, basic husbandry, and feeding logistics. ECHO International Agriculture Conference 2019 Presentation.
Barry, T. (Ph.D. dissertation). 2004. Evaluation of the Economic, Social, and Biological Feasibility of Bioconverting Food Wastes With the Black Soldier Fly (Hermetia illucens). University of Texas, 176 pp.
Bruno, D. M. Bonelli, F. De Filippis, I. Di Lelio, G. Tettamanti, M. Casartelli, D. Ercolini, and S. Caccia. 2019. The intestinal microbiota of Hermetica illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Applied and Environmental Microbiology 85(2): e01864-18.
Castillo, C., and J. Hernández. 2023. Insects in ruminant nutrition as an urgent measure in the light of the scarcity of raw feedstock. Research in Veterinary Science, Vol.155:124-125.
Chalermliamthong, S. and P. Trail. 2021. Establishing a scaled-up black soldier fly system. ECHO Asia Note 47:1-7.
Chen, S., W. Liao, C. Liu, Z. Wen, R.I. Kincaid, J.H. Harrison, D.C. Elliott, M.D. Brown, A.E. Solana, and D.J. Stevens. 2003. Value-added chemicals from animal manure. Environmental Molecular Sciences Laboratory, Pacific Northwest Laboratory. https://doi.org/10.2172/15009485.
Cheng, V., A.K. Shoveller, L.A. Huber, and E.G. Kiarie. 2023. Comparative protein quality in black soldier fly larvae meal vs. soybean meal and fish meal using classical protein efficiency ratio (PER) chick growth assay model. Poultry Science, Vol. 102 (1)102255.
Chia, S.Y., C.M. Tanga, F.M. Khamis, S.A. Mohamed, D. Salifu, S. Sevgan, K.K. M. Fiaboe, S. Niassy, J.J.A. van Loon, M. Dicke, and S. Ekesi. 2018. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLOS ONE 13(11): e0206097.
de Souza Vilela, J., N.M. Andronicos, M. Kolakshyapati, M. Hilliar, T.Z. Sibanda, N.R. Andrew, R.A. Swick, S. Wilkinson, and I. Ruhnke. 2021. Black soldier fly larvae in broiler diets improve broiler performance and modulate the immune system. Animal Nutrition. 7(3):695-706.
Dortmans B.M.A., J. Egger, S. Diener, and C. Zurbrügg. 2021. Black Soldier Fly Biowaste Processing - A Step-by-Step Guide, 2nd Edition Eawag: Swiss Federal Institute of Aquatic Science and Technology.
Fanatico, A.C., K. Arsi, I. Upadhyaya, J. Morales Ramos, D. Donoghue, and A.M. Donoghue. 2018. Sustainable Fish and Invertebrate Meals for Methionine and Protein Feeds in Organic Poultry Production. Journal of Applied Poultry Research. Vol. 27(4)437-448.
Fawole, F.J., S.N. Labh, M.S. Hossain, K. Overturf, B.C. Small, T.L. Welker, R.W. Hardy, and V. Kumar. 2021. Insect (black soldier fly larvae) oil as a potential substitute for fish or soy frass derived from black soldier fly larvae treatment of biodegradable wastes. a critical review and future perspectives. Animal Nutrition Vol. 7 (4):1360-1370.
Goddard, J. 2003. Physician’s Guide to Arthropods of Medical Importance, 4th Edition. CRC Press LLC. Boca Raton, Florida.
Gorrens, E. N. Van Looveren, L. Van Moll, D. Vandeweyer, D. Lachi, J. De Smet, and L. Van Campenhout. 2021. Staphyloccoccus aureus in substrates for black soldier fly larvae (Hermetia illucens) and its dynamics during rearing. Food Microbiology 9(3): e02183-21.
Green, T. 2013. BSF & food scrap: putting the leachate to good use. DipTerra LLC- Specializing in Recycling with Black Soldier Fly Technologies. https://www.dipterra.com/blog/bsf-food-scrap-putting-the-leachate-to-good-use
Hong, J. and Y.Y. Kim. 2022. Insect as feed ingredients for pigs. Animal Bioscience 35(2):347-355.
Ipema, A.F., Gerrits, W.J.J, Bokkers, E.A.M. Kemp, B. and J.E. Bolhuis. 2021. Live black soldier fly larvae (Hermetia illucens) provisioning is a promising environmental enrichment for pigs as indicated by feed- and enrichment-preference tests. Applied Animal Behaviour Science. Vol. 244:105481.
Jayanegara, A., B. Novandri, N. Yantina, and M. Ridla. 2017. Use of black soldier fly larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: an in vitro rumen fermentation study. Vet World 10(12):1439-1446.
Kar, S. K., D. Schokker, A.C. Harms, L. Kruijt, M.A. Smits, and A.J.M. Jansman. 2021. Local intestinal microbiota response and systemic effects of feeding black soldier fly larvae to replace soybean meal in growing pigs. Scientific Reports 11:15088 https://doi.org/10.1038/s41598-021-94604-8
Lamin, S. A. Abrar, Arwinsyah, M. Kamal, and A.N. Sipahutar. 2022. The effect of some attractive medio on the number of marriage partners, eggs weight and lifetime of black soldier fly (Hermetia illucens L.) Biovalentia: Biological Research Journal 8(2):151-155.
Li, S., H. Ji, B. Zhang, J. Tian, J. Zhou, and H. Yu. 2016. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture Vol. 465:43-52.
Lopes, G., J. W.H. Yong and C. Lalander. 2022. Frass derived from black soldier fly larvae treatment of biodegradable wastes: a critical review and future perspectives. Waste Management 42:65-76.
Moula, N., M. Scippo, C. Douny, G. Degand, E. Dawans, J. Cabaraux, J. Hornick, R.C. Medigo, P. Leroy, F. Francis, and J. Detilleux. 2018. Performances of local poultry breed fed black soldier fly larvae reared on horse manure. Animal Nutrition. 4(1)73-78.
Müller, A., S. Wiedmer, and K. Michael. 2019. Risk evaluation of passive transmission of animal parasites by feeding of black soldier fly (Hermetia illucens) larvae and prepupae. Journal of Food Protection 82(6):948-954.
Nguyen, T.T.X., J.K. Tomberlin, and S. Vanlaerhoven. 2013. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. Journal of Medical Entomology 50(4):898-906.
Nyangena, D.N. C. Mutungi, S. Imathiu, J. Kinyuru, H. Affognon, S. Ekesi, D. Nakimbugwe, and K.K.M. Fiaboe. 2020. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods 9:574 doi:10.3390/foods9050574.
Palma, L. J. Fernandez-Bayo, D. Niemeier, M. Pitesky, and J.S. VanderGheynst. 2019. Managing high fiber food waste for the cultivation of black soldier fly larvae. NPJ Science of Food 3:15 doi:10.1038/s41538-019-0047-7.
Parodi, A., W. J.J. Gerrits, J. J.A. Van Loon, I. J.M. De Boer, A. J.A. Aarnink, H., and H.E. Van Zanten. 2021. Black soldier fly reared on pig manure: bioconversion efficiencies, nutrients in the residual material, greenhouse gas and ammonia emissions. Waste Management. Vol. 126:674-683.
Rehman, K. ur, C. Hollah, K. Wiesotzki, R. ur Rehman, A. Ur Rehman, J. Zhang, L. Zheng, T. Nieaber, V. Heinz, and K. Aganovic. 2022. Black Soldier Fly, Hermetia illucens as a potential innovative and environmentally friendly tool for organic waste management: a mini-review. Waste Management & Research: The Journal for a Sustainable Circular Economy 41(1):81-97.
Romano, N., A. Powell, S. Islam, H. Fischer, N. Renukdas, A. Kumar Sinha, and S. Francis. 2022. Supplementing aquaponics with black soldier fly (Hermetia illucens) larvae frass tea: Effects on the production and composition of sweet potato slips and sweet banana peppers. Aquaculture. Vol. 555:738160.
Ruhnke I., C. Normant, D.L.M. Campbell, Z. Iqbal, C. Lee, G.N. Hinch, and J. Roberts. 2018. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Animal Nutrition. Vol.4(4):452–460.
Sheppard, D.C., J.K. Tomberlin, J.A. Joyce, B.C. Kiser, and S.M. Sumner. 2002. Rearing methods for the black soldier fly (Diptera: Stratiomydae). Journal of Medical Entomology 39(4):695-698.
Shishkov, O., M. Hu, C. Johnson, and D.L. Hu. 2019. Black soldier fly larvae feed by forming a fountain around food. Journal of the Royal Society Interface 16(151):20180735
Soomro, A.A. M. Cai, Z.A. Laghari, L. Zheng, K. ur Rehman, X. Xiao, S. Hu, Z. Yu, and J. Zhang. 2021. Impact of heat treatment on microbiota of black soldier fly larvae reared on soybean curd residues. Journal of Insects as Food and Feed 7(3):329-343.
Smil, V. 2002. Eating meat: evolution, patterns, and consequences. Population and Development Review 28(4):599-639.
Tomberlin, J.K., P.H. Adler, and H.M. Myers. 2009. Development of black soldier fly (Diptera: Stratiomyidae) in relation to temperatura. Environmental Entomology 38(3):930-934.
Tomberlin, J.K., D. Craig Sheppard, and J.A. Joyce. 2002. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Annals of the Entomological Society of America 95(3):379-386.
Wong, A. 2020. Black soldier fly of the Frangipani Langkawi Organic Farm. ECHO Asia Notes 41:7-9.
Xiao, Y., W. Geng, Y. Yang, X. Wang, and X. Xu. 2020. Study on the difference of transformation of livestock and poultry feces by black soldier fly. IOP Conference Series: Earth and Environmental Science 450 (1): 012112.
Yakti, W., M. Müller, M. Klost, I. Mewis, D. Dannehl, and C. Ulrichs. 2023. Physical properties of substrates as a driver for Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae growth. Insects 14, 266 https://doi.org/10.3990/insects14030266.
Yildirim-Aksoy, M., R. Eljack, C. Schrimsher, and B.H. Beck. 2020. Use of dietary frass from black soldier fly larvae, Hermetia illucens, in hybrid tilapia (Nile x Mozambique, Oreocromis niloticus x O. mozambique) diets improves growth and resistance to bacterial diseases. Aquaculture Reports. Vol. 17:100373.
Zarantoniello, M., A. Zimbelli, B. Randazzo, M. Delli Compagni, C. Truzzi, M. Antonucci, P. Riolo, N. Loreto, A. Osimani, V. Milanović, E.Giorgini, G. Cardinaletti, F. Tulli, R. Cipriani, G. Gioacchini, and I. Olivotto. 2020. Black Soldier Fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 518: 734659.
Zhang, Y. X. Xiao, O. Elhag, M. Cai, L. Zheng, F. Huang, H.R. Jordan, J.K. Tomberlin, S-H Sze, Z. Yu., and J. Zhang. 2022. Hermetia illucens L. larvae-associated intestinal microbes reduce the transmission risk of zoonotic pathogens in pig manure. Microbial Biotechnology 15(10):2631-2644.
Zhao, J., K. Kawasaki, H Miyawaki, H. Hirayasu, A. Izumo, S. Iwase, K. Kasai. 2022. Egg quality and laying performance of Julia laying hens fed with black soldier fly (Hermetia illucens) larvae meal as a long-term substitute for fish meal. Poultry Science 101(8):101986.
Zulkifli, N.F.N.M., A.Y. Seok-Kian, L.L. Seng, S. Mustafa, Y.S. Kim, and R. Shapawi. 2022. Nutritional value of black soldier fly (Hermetia illucens) larvae processed by different methods. PLOS One 17(2):e0263924.