[Editor’s Note: For questions, comments, or personal experience on this topic visit ECHOcommunity Conversations: African Swine Fever Virus]
The African Swine Fever Virus and its Effects on Global Pork Production
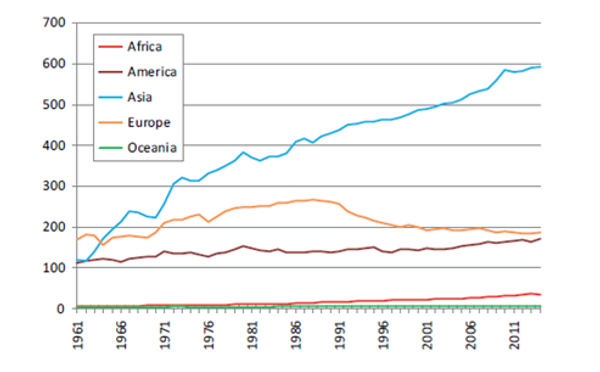
Figure 1. Number of pigs globally (x 1,000,000) by region from 1961 to 2014. (FAOSTAT, 2016).
As global prosperity has risen, so too has demand for animal protein. Pork has become the most consumed meat of terrestrial animals at 37% of total global meat consumption (Beltrán-Alcrudo et al., 2017). Pork production most drastically increased in Asia (Figure 1), accounting for as much as 55% of global pork production in 2018 (FAOSTAT, 2018). Global gross pork production value has hovered around 300 billion USD since 2011 (FAOSTAT, 2020). In 2018 this all changed with the onset of the African Swine Fever Virus (ASFV) in Asia. This deadly virus has decimated pig populations and brought significant reductions in global pork production and pork protein consumption. It is estimated that upwards of 25% of the world’s pig population has succumbed to ASFV over the past two years (Niederwerder et al., 2020).
Origin, Description, and Distribution of ASFV
African Swine Fever Virus is the sole member of the Asfarviridae family of viruses. As its name suggests, ASFV originated on the African continent where it remains widespread. Soft-bodied ticks (Ornithodoros genus) and other members of the swine family (Suidae) host the virus and facilitate its spread. Domestic swine and wild swine (Sus scrofa) are highly susceptible to the disease. Many native wild swine are generally asymptomatic but do act as hosts and spreaders for the virus (OIE, 2019).
Most of the 32 isolates of the virus are spread across Africa. In 1957, one of these isolates was introduced into Portugal from West Africa, where it spread across Europe, parts of the Caribbean, and Brazil. Eradication of the virus in these countries was achieved, but it persisted until the 1990s in Spain and Portugal (Beltrán-Alcrudo et al., 2017). The current outbreak in Europe and Asia began in 2007 when another isolate of ASFV entered the nation of Georgia from southeastern Africa and gradually spread across Europe (Beltrán-Alcrudo et al., 2017). In 2017, Russia experienced an outbreak of ASFV which then spread to Northern China in 2018. Since 2018, it has spread throughout China and much of Southeast Asia (Schneider, 2020).
Transmission and Diagnosis
Understanding the Transmission of ASFV
In Africa, the virus is spread between ticks and wild boar hosts, from direct contact with infected domestic pigs, and through infected materials accidentally introduced by humans. In Europe, wild boars play a prominent role in the spread of the disease (Table 1), while in Asia, the virus spreads largely from domestic pig to domestic pig and from humans spreading infected material (Beltrán-Alcrudo et al., 2017). This means that with proper education and community-enforced biosecurity, total prevention, and eradication of the disease in Asia is possible. This was the case for the outbreaks of the late 1950s in Asia.
| Swine | Wild boar | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Outbreaks | Susceptible | Cases | Losses | Outbreaks | Susceptible | Cases | Total Outbreaks | Total Cases |
| Africa | 128 | 213795 | 61459 | 85539 | 128 | 61459 | |||
| Asia | 9928 | 8107951 | 115309 | 6733791 | 631 | NA | 1121 | 10559 | 116430 |
| Europe | 4271 | 1859480 | 625269 | 1383372 | 17307 | NA | 29513 | 21578 | 654809 |
| Total | 14327 | 10181226 | 802064 | 8202702 | 17938 | NA | 30634 | 32265 | 832698 |
Animals begin to show signs of infection between 4 and 19 days after transmission. Pigs can spread the virus up to two days prior to showing symptoms. An animal that lives long enough (perhaps due to a less virulent strain) can be infectious for more than 70 days after the initial infection. African Swine Fever Virus is highly lethal and resilient; it spreads through swine bodily fluids such as blood, saliva, tears, nasal secretions, urine, feces, and genital tract excretions. Blood carries a notably large amount of the virus. Infection can spread through direct contact with other infected pigs, or by contacting or ingesting infected material. Infected material could be anything carrying even small amounts of bodily fluids containing the virus. The virus is stable in a wide range of environments (Table 2). Persisting at pH levels between 3.9 and 13.4 and surviving freeze-thaw cycles. The virus can survive months in meat, blood, and on contaminated surfaces. Even curing meats, such as in pork sausage, will not kill the virus and can actually extend the life of the virus (Beltrán-Alcrudo et al., 2017). This resilience has far reaching implications, among them a concern for the movement of people and vehicles in and around farms. It should be noted that there is no evidence to suggest that the ASF virus affects humans and infect meat products are not known to cause harm.
| Item | ASFV survival time |
|---|---|
| Meat with and without bone and ground meat | 105 days |
| Salted meat | 182 days |
| Cooked meat (minimum of 30 minutes at 70°C) | 0 |
| Dried meat | 300 days |
| Smoked and deboned meat | 30 days |
| Frozen meat | 1000 days |
| Chilled meat | 110 days |
| Offal | 105 days |
| Skin/Fat (even dried) | 300 days |
| Blood stored at 4°C | 18 months |
| Feces at room temperature | 11 days |
| Putrefied blood | 15 weeks |
| Contaminated pig pens | 1 month |
| Source: adapted from Scientific Opinion on African swine fever, EFSA Journal, 2010; 8(3):1556. The times given reflect the known or estimated maximum duration and will depend strongly on environmental temperature and humidity. |
|
Diagnosis
Diagnosis of ASVF by visual assessment of symptoms alone can be difficult. The most telling sign is sudden increases in death in both genders. Symptoms vary widely and depend upon the virulence of the virus, swine breed, exposure dosage, route of exposure and the endemic nature of the virus in the area. Less deadly forms of the virus will have mortality rates below 60% with some as low as 10%. However, Asia currently hosts very virulent strains with mortality rates can be as high as 100% (Beltrán-Alcrudo et al., 2017).
Animals showing severe (paracute) symptoms will typically have a high fever of 41-42°C (3-4°C higher than normal; Birmingham & Quesenberry, 2000) and will likely die several days before showing any clinical symptoms. Slightly less severe (acute) symptoms delay death long enough to show symptoms but still result in 90-100% death in herds. Animals will suffer from a fever of 40-42°C, increased respiratory rates, loss of appetite, and sluggish behavior. Death typically occurs as early as 6 to 11 days after symptoms appear.
Pigs infected with ASFV can exhibit any of the following symptoms:
- Bluish-purple areas and internal or external bloodied areas (spot-like or extended) on the ears, abdomen, and/or hind legs
- Ocular (ear) and nasal (snout) discharge
- Reddening of the skin of the chest, abdomen, perineum, tail, and legs
- Constipation or diarrhea, which may progress from mucoid to bloody
- Vomiting
- Abortion by pregnant sows at all stages of pregnancy
- Bloody froth from the nose/mouth and a discharge from the eyes
- The area around the tail may be soiled with bloody feces
The Food & Agriculture Organization “Manual for ASFV Detection and Diagnosis” (FAO, 2010) offers the following list of ASFV symptoms found in postmortem examinations of infected pigs. Usually several are present simultaneously (Figure 2):
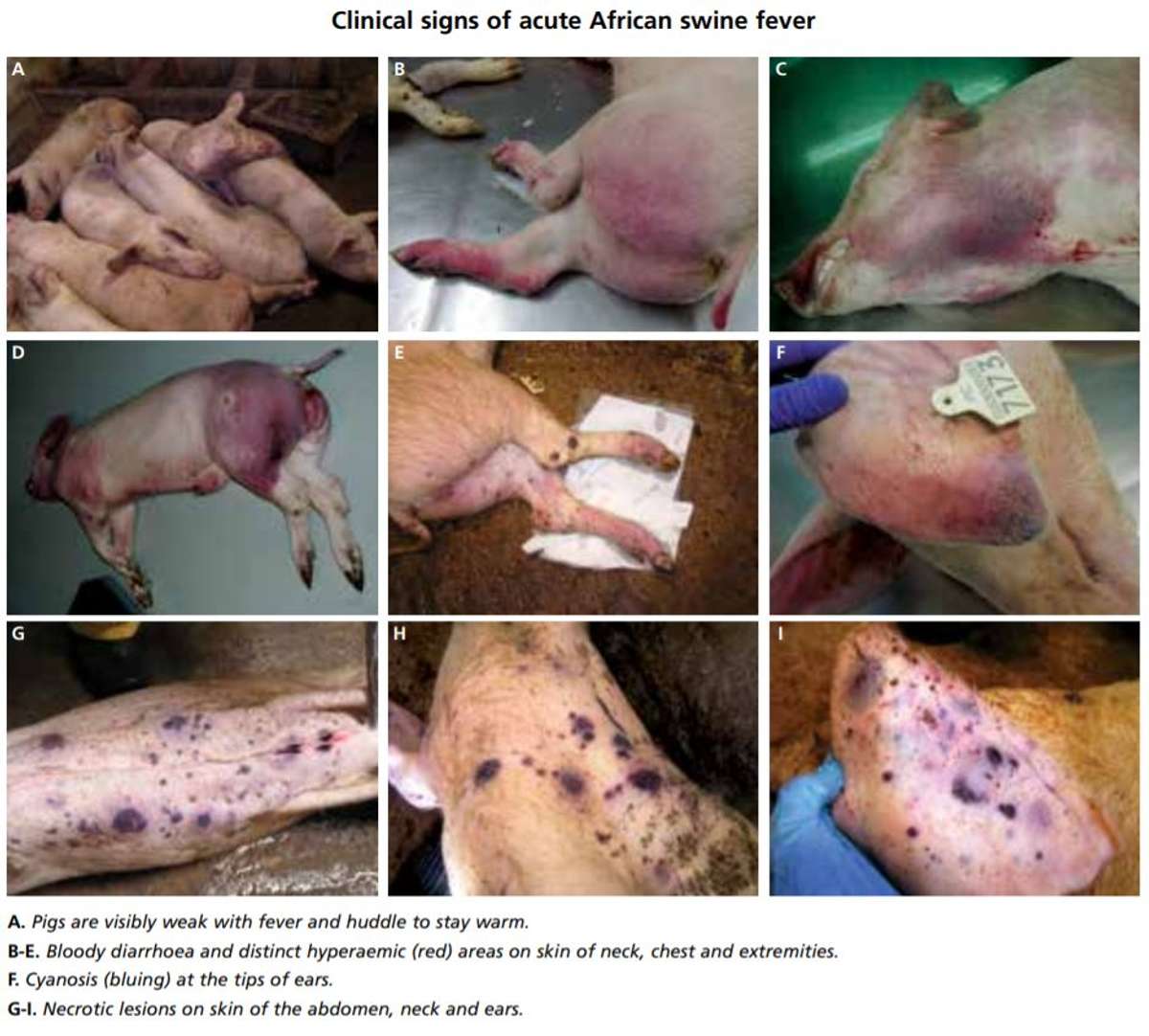
Figure 2. Clinical symptoms of acute African Swine Fever (Belrán-Alcrudo et al., 2017).
- Hemorrhages under the skin
- Excess of fluids in the heart (hydropericardium with yellowish fluid) and body cavities (hydrothorax, ascites)
- Petechiae (pinpoint bleeding wounds) on the heart’s surface (epicardium), urinary bladder, and kidneys (on the cortical and renal pelvis)
- Lungs may present congestion and petechiae, with froth in the trachea and bronchus, and severe alveolar and interstitial pulmonary oedema (dropsy)
- Petechiae, ecchymoses (larger hemorrhages), and excess clotted blood in the stomach and small and large intestines'
- Hepatic (liver) congestion and hemorrhages in the gall bladder
**It is not recommended to perform postmortem examinations outside the presence of a trained veterinarian or livestock authority, due to the risk for exposing the farm area to undue fluids containing viral contaminants.
Diagnosis of ASFV is difficult as it is easily confused with several other diseases and therefore cannot be definitively diagnosed until laboratory tests confirm ASFV. Classical swine fever, swine erysipelas, poisoning, salmonella, and other septicemic conditions are easily confused with ASFV. It is recommended that producers not rely on their own prognosis until confirmed by a veterinary professional.
Coping with the Disease
Prevention & Control
At the writing of this article (2021), no vaccine exists for ASFV, though efforts are well underway to synthesize one. If and when a vaccine becomes available, it may become the best course of preventative action. In the absence of an effective vaccine, preventing the spread of ASFV with persistent biosecurity and eradication of the disease upon detection are the keys to mitigating its impacts.
Every farm is different and will address threats of infection differently. The threat of such a deadly virus demands a thorough biosecurity plan that all staff can be made aware of and held accountable to. Plan development will benefit from asking staff and others to help in identifying possible avenues of infection. Prevention and control begin with all those involved in farm operations gaining an awareness of the severity of ASFV and its mechanisms of spread. Humans are assumed to be a primary spreader of ASFV. They easily carry infected material on boots, clothing, or other materials so group awareness is essential.
A biosecurity plan can be as simple as making a list of disease threats, their entry points, and subsequently developing approaches to eliminate or minimize those threats. The FAO suggests three steps for biosecurity: (1) segregation, (2) sanitation, and (3) disinfection (FAO, 2010).
Segregation
Segregation is physically isolating livestock from any possible contaminants. Contamination can enter the farm from many sources such as other pigs and pig products; pig bedding and pig manure; water runoff from nearby operations; pig semen; boots or clothing, feed, vehicles, or other animals. Keeping pigs segregated from outside pig herds is a best practice, as is restricting access to pigs to essential personnel only. Employee interaction with swine outside the farm should be discussed and minimized. A prudent measure is to have separate footwear and clothing for inside and outside the pig area. To minimize chance of infection, have all employees start the day working directly with pigs and then move further away from them to other potentially infected areas or pigs in quarantine. Not in the reverse order.
Sanitation
Before coming into contact with pigs, any person or object should be properly cleaned. This is different from using a disinfectant. Often a disinfectant will not be able to penetrate something like mud or manure, which could then be the entry point for ASFV. Many pig operations around the world change footwear, clothing, and require staff to shower before entering and exiting the area where pigs are kept. Tire tread can harbor infected materials so washing and scrubbing them to remove lose material is important.
Disinfection
After physical cleaning, a disinfectant approved for ASFV purposes should be used. The African Swine Fever Virus is densely encapsulated and difficult to kill with disinfectants. All disinfectants require significant contact time to kill the virus. It is therefore important not to rush the disinfection step and to thoroughly clean before disinfecting to maximize contact time. Chlorine is a viable disinfectant (0.5% for 30 min), as well as iodine, ether, chloroform, formalin (30 min) and caustic soda (NaOH; 8/1000 for 30 min; OIE, 2019). A foot bath of disinfectant can provide sanitation for boots before entering the farm. Stepping in lime has been recommended after a foot bath to raise the pH to greater than the virus can survive. A bath of disinfectant and lime can be set up for vehicles as well but must be long enough that the entire circumference of the tire is covered. Washed tires should be allowed to dry before entering the disinfectant bath (Dr. P. Quesenberry, Personal Communication, Sept 3, 2020).
| Potential Sources of Contamination | Action Steps |
|---|---|
| Other Pigs | Confine pigs and keep new animals quarantined in a separate location for 30 days; cull and bury pigs showing ASFV symptoms. |
| Food Scrap Feeding | This is discouraged. It should be avoided unless food has been cooked at 70°C for at least 30 minutes. |
| Other Animals such as Rodents | Exterminate, minimize open food sources, and close entry points as much as possible. |
| Contaminated Shoes or Clothing | Change clothes before entering and exiting facility, clean and sanitize, and minimize entry and exit from pig areas. Work from clean to potentially dirty areas. |
| Contaminated Vehicles | Minimize vehicle proximity to animals, scrub tires, and drive through sanitizing dip. |
| Feed | Only buy feed from reputable sources. Check for signs of rodent damage and destroy contaminated feed. |
| Water | Avoid open water sources when possible. Divert water outflow away from the farm. |
Heat treatment, if done properly, is an effective method of deactivating the virus. One study from 1967 found ASFV could survive 11-22 days at 37°C but at higher temperatures was inactivated more quickly. It survives only 1 hour at 56°C and 15 minutes at 60°C (Mazur-Panasiuk et al., 2019). The FAO recommends that potentially contaminated feed be cooked 30 minutes at 70°C (Beltrán-Alcrudo et al., 2017). This has implications for swill feeding (feeding of food scraps), currently a major spreader of the virus.
Community Prevention
- Pig confinement
- Exclusion of free ranging rodents, dogs, and other animals from pig areas
- Keeping non-essential people out of pig pens
- Keeping shoes clean of manure
- Managing stud boars for biosecurity
- Not moving pigs in or out of local area (especially pigs dead from disease)
- Purchasing, storing, and selling of feed in a biosecure manner.
It will be impossible to block every possible entry point of ASFV into a community, but if a community can agree upon some basic guidelines, spread of the virus may be prevented or sufficiently reduced. Of first importance is agreeing to confine pigs. A recent study looked at first steps for managing ASFV in Timor-Leste where very little biosecurity had been practiced. This study showed significantly greater loss amongst non-fenced pigs than amongst fenced pigs (Barnes et al., 2020). Such community-level discussions can involve small or large groups and may take many formats. Each community will be different and will be presented with different challenges, but conversation and consensus are key in keeping ASFV out of communities and slowing the spread of disease. 1
ASFV has easily spread from country to country in Asia. Smuggling of pig products across borders is suspected to be a primary vector for this spread, and many governments have taken this very seriously. To coordinate efforts, be aware of and follow local government regulations regarding reporting and handling of infected pigs.
Opportunities for Smallholder Operations
Smallholders remain a major contributor to pork production in Asia. “Smallholders” or “small-scale operations” are typically defined as operations keeping anywhere from 1 to 100 pigs (Nga et al., 2015; FAO, 2010). A 2015 study in Vietnam found that smallholders account for approximately 80% of pork consumed domestically (Nga et al., 2015). In China pork prices have spiked because of loss of over half of the country’s pig population (Shneider, 2020). This rise in price, as is the case in many other Southeast Asian countries, may provide good opportunity for operations that can remain ASFV free, particularly for producers that sell locally. International restrictions have already been put in place for some countries (Shen & Look, 2020) making local markets a more stable source for pork.
The rise in pork prices may also open opportunities for chicken, fish, beef, and other livestock sales to fill the protein gap. Many small producers may consider further diversifying animal production to minimize risk and take advantage of high protein demand in the market.
Conclusion
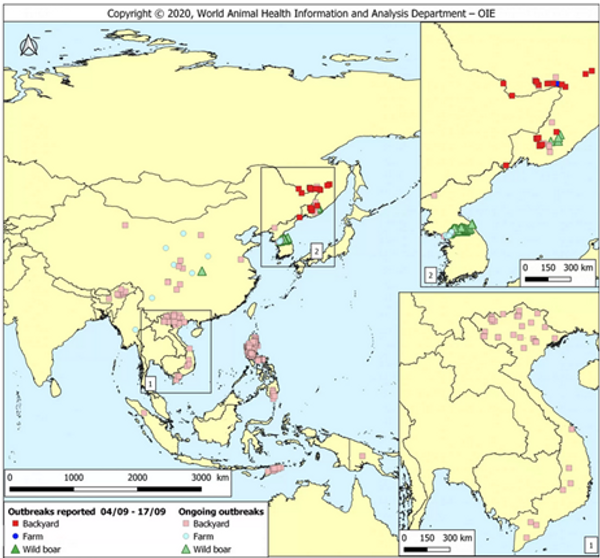
Figure 3. OIE report on outbreak source and spread from Sept 2020 (World Animal Health Information & Analysis Department – OIE).
African Swine Fever Virus poses one of the greatest challenges faced by small-scale Asian farmers in recent memory, bringing with it high potential for loss and altered livelihoods. Study after study identifies smallholder and backyard farmers as primary spreaders of ASFV, and particularly vulnerable to its effects (Nga et al., 2015; FAO, 2020; OIE, 2020; Barnes et al., 2020). Several outbreaks in the region have been classified as “backyard” case by their respective governments (Figure 3), leaving room for the blame and potential for government aid and legislation to remain in favor of large conventional pig operations more capable of implementing adequate biosecurity measures. Regulations have the potential to effectively eliminate the smallholder portion of the pork industry if action is not taken. It is important, now more than ever, for communities that value and rely on small-scale integrated pig operations to make community level decisions to stop the spread of ASFV.
References
Barnes, T. S., Morais, O., Cargill, C., Parke, C. R., Urlings, A. (2020). First Steps in Managing the Challenge of African Swine Fever in Timore-Leste. One Health. Vol (10). https://doi.org/10.1016/j.onehlt.2020.100151.
Beltrán-Alcrudo, D., Arias, M., Gallardo, C., Kramer, S. & Penrith, M.L. (2017). African Swine Fever: Detection and Diagnosis – A Manual for Veterinarians. FAO Animal Production and Health Manual No. 19. Food and Agriculture Organization of the United Nations (FAO).
Birmingham, M., Quesenberry P. (2000). Where there is no Animal Doctor. Seattle, WA. USA: Christian Veterinary Mission.
Cunningham, M., Latour, M. A., & Acker, D. (2005). Animal Science and Industry. Upper Saddle River, NJ: Pearson Prentice Hall.
Food and Agriculture Organization of the United Nations. (2020). FAOSTAT Statistical Database. Retrieved October 10, 2020, from http://www.fao.org/faostat/en/#data/QV/visualize
Food and Agriculture Organization of the United Nations. (2010). Good Practices for Biosecurity in the Pig Sector- Issues and Options in Developing and Transition Countries. FAO Animal Production and Health Manual. Retrieved on Sept 12, 2020, from: http://www.fao.org/3/a-i1435e.pdf
Iowa State University. (2020). African Swine Fever Technical Fact Sheet. The Center for Food Security and Public Health. Retrieved August 29, 2020, from http://www.cfsph.iastate.edu/DiseaseInfo/disease.php?name=african-swine-fever&lang=en
Mazur-Panasiuk, N., Żmudzki, J., Woźniakowski, G. (2019). African Swine Fever Virus – Persistence in Different Environmental Conditions and the Possibility of its Indirect Transmission. Journal of Veterinary Research. http://doi.org/10.2478/jvetres-2019-0058
Nga, N.T.D, Lapar, L., Unger, F., Hung, P. V., Ha, D. N., Huyen, N. T. T., Long, T. V., Be, D. T. (2015). Household Pork Consumption and Behavior in Vietnam: Implications for Pro-Smallholder Pig Value Chain Upgrading. Conference on International Research on Food Security, Natural Resource Management and Rural Development. Retrieved August 21, 2020 from https://www.researchgate.net/publication/302904120 Household pork consumption behavior in Vietnam Implications for pro-smallholder pig value chain upgrading
Niederwerder, M. C., Dee, S., Diel, D. G., Stoian, A. M., Constance, L. A., Olcha, M., Petrovan, V., Patterson, G., Cino-Ozuna, A.G., & Rowland, R. R. (2020). Mitigating the risk of African Swine Fever Virus in Feed with Anti-Viral Chemical Additives. Transboundary and Emerging Diseases. https://doi.org/10.1111/tbed.13699
OIE. (2020). Global Situation of African Swine Fever. Retrieved August 16, 2020, from https://www.oie.int/en/disease/african-swine-fever/
OIE. (2019). African Swine Fever. Retrieved August 1, 2020, from https://www.oie.int/en/disease/african-swine-fever/
Schneider, M. (2020, February 17). The Pork Fix: African Swine Fever and the Opportunity of Crisis in China’s Pork Industry. [Video file]. Retrieved August 01, 2020, from https://ecommons.cornell.edu/handle/1813/69876
Shen, F., Look, C. (2020). African Swine Fever: China Bans German Pork Over Fears of Deadly Hog Disease. Bloomberg Business. Retrieved September 12, 2020, from https://www.bloomberg.com/news/articles/2020-09-12/china-bans-german-pork-imports-over-swine-fever-cases